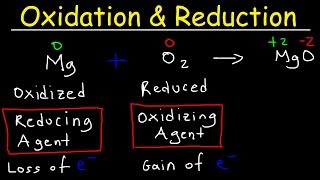
Electrochemistry Review - Cell Potential & Notation, Redox Half Reactions, Nernst Equation
Вставка
- Опубліковано 6 лют 2025
- This electrochemistry review video tutorial provides a lot of notes, equations, and formulas that you need to pass your next chemistry test / exam. It’s a nice video that provides an introduction or overview of electrochemistry including the most important fundamental topics and concepts. It contains plenty of examples and practice problems.
Electrochemistry - Free Formula Sheet:
bit.ly/3NLeylq
Chapter 17 - Video Lessons:
www.video-tuto...
Chemistry 2 Final Exam Review:
• General Chemistry 2 Re...
_______________________________
Intro to Galvanic & Voltaic Cells:
• Introduction to Galvan...
How To Draw Galvanic Cells:
• How To Draw Galvanic C...
Standard Reduction Potentials:
• Standard Reduction Pot...
Cell Potential Problems:
• Cell Potential Problem...
Cell Notation Problems:
• Cell Notation Practice...
___________________________________
Concentration Cells:
• Concentration Cells & ...
Cell Potential & Gibbs Free Energy:
• Cell Potential & Gibbs...
Cell Potential & Equilibrium K:
• Equilibrium Constant K...
Nernst Equation:
• Nernst Equation Explai...
Electrolysis of Water:
• Electrolysis of Water ...
_____________________________________
Electrolysis of Sodium Chloride:
• Electrolysis of Sodium...
Electrolysis & Electroplating Problems:
• Electrolysis & Electro...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
SAT Chemistry Subject Test Review:
• SAT Chemistry Subject ...
Carbon -14 Dating:
• Carbon 14 Dating Probl...
Beer Lambert's Law:
• Beer Lambert's Law, Ab...
______________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tuto...








Electrochemistry - Free Formula Sheet: bit.ly/3NLeylq
Chapter 17 - Video Lessons: www.video-tutor.net/electrochemistry.html
Great videos
Gotta really love it tbh
I got into Medical School because of you!
The videos you have here on youtube are the best out there!
Thank you so much for what you do!
congrats!
That’s very good stuff bro
cries
congratulations!
Read your bible! (KJV, preferably) ♥
I watch 1 video of this guy and I instantly turn into the guy other people ask for help from on the morning of the exam
Already 3 min in and confusing concepts become so clear.
The example of a laptop plugged in or not is SO easy to follow
-wish my professor could talk sense like this:)
Whether it be physics or chemistry, you're really great at teaching topics when my teachers can't. Thanks for the last minute prep before AP exams man.
I am here to thank you)) a few months ago, I was almost zero in chemistry, but with the help of your videos I could pass my general chemistry exam from the first attempt. even if I got the minimum passing score, I still did it, I can't believe it. I thought I will take a re-exam... thanks a lot sir🙏 to everyone reading this: do not be lazy, do not leave everything on the last days. good luck everyone)
damn i am writing tomorrow, looks like ill just have to take the re-exam
@@starboii568 hey, just try to do your best
@@lazizand thanks bro
@@starboii568 so, how was your exam? hope you did well
@@lazizand i passed all my subjects except for chemistry 😭😭. I need 3 marks to pass.
I CAN'T STOP SMILING!
In 7 minutes you explained and introduced a technique a text book took 30 PAGES and didn't reach to a 100th of what you surmounted to.
Deep and Grateful THANKS!!!!
I am now in 3rd year Bsc Chemistry and every time you are my preferred lecturer, am getting my degree from you!
Another Zambian 😅
Your videos have help me so much for the last year. Thank you so much for really taking your time and and providing so many examples. You have helped me on every test I've taken for my past 3 chem classes. I appreciate it very much.
You're very welcome
You're the reason I can cram a whole semester's worth of knowledge in one speed run through your videos and pass my gen chem class! Thank you!
Lol😂
me too
like a boss
I got admitted into UCLA because of youu!!!!! 🙏🏼
Bit of a necropost, but congratulations on your admission!
@@MrAblublublublu Bit of a necropost, but that's friendly of you!!
Out in the yeeard bit of a necropost, but great!!
@@jacklabrier8039 Bit of a necropost, but DEATH TO ALL IS NEAR
congratulations!!!
I just want to say. There has never been a better teacher.
I'm in my third year of a chemistry degree because of this guy. I used to hate chem in highschool until I started understanding it. Nobody put in the time to help me but I put the time into myself and ochem tutor is a real one for that.
Me see organic chemistry tutor, me click ,me get good marks, me happy.
Coronavirus shuts down your university + forced to do online school + didn't know what the hell was going on to begin with when you were in school = finding this channel on UA-cam
true
Got an exam in three hours 😭😭I don’t know what I’d do without your channel
I also watched this 2 hours before my exam ❇️
Am in your shoes now
I understand better now the things my teacher has been trying to teach a whole semester thanks.🤝
You sir are doing a great thing. Thanks to you I've aced three chemistry classes so far.
you've really help me to understand science in all its branches, we deeply appreciate you sir for the knowledge you share ...ive been watching your lecture videos. i find that i understand you more than my own lecturers continue helping people ..Steve (Zambia) ...
I knew nothing about electrochem before this vid and my AP test is tomorrow. God bless u
I like your videos because you explain all the steps, but do not repeat yourself or waste words. Good flow.
I LOVE YOU ORGANIC CHEMISTRY TUTOR!
At 34.26 minutes of video,
∆G = ∆G^o - RT lnQ
I have 2 textbook here of Gen Chemistry saying that
∆G = ∆G^o + RT lnQ
^o is to superscript, to set delta G in standard state
I am learning a lot from you but I like to clarify this formula..Thank you.
The textbooks are:
Fundamentals of Chemistry, 4th Ed. by Frank Brescia etc. page 311
Principles of General Chemistry, 3rd edition by Martin S. Silberberg, page 676
You r right bro ; he forgot to see his mistake
At 58:26, If you multiply the reaction by 2, wouldn't the half reaction then be 1.54V? And the new total would be 1.77V?
edit: 1:03:12 The answer is no.
If the reaction is multiplied by a coefficient the cell potential won't be affected or altered.
It is only altered when the reaction is reversed.
i am currently on crystal field theory and JG has no video on it. Not happy. but he has this desperately needed video. Yahh! Happy.
I believe without you half or almost all of sl students would have gotten problems in their study. Thanks for keeping us informed
bless ur kind-hearted soul, sir. that is all
I couldnt understand this subject anywhere else but here ❤❤ love how you tell us the logic behind everything
What an awesome lesson! you explain all of these concepts through a simple and intuitive way! Congrats
Watching this 40 minutes before my final!
Me too 😭
@@yasanlungu5594 how’d it go yasan
@@Goos836 pretty well 🤡
me too i really decided to take general chem 2 during a 6 week summer course o god.
You’ve always been the best💯 Thank you for always making my studies easy🙏 Don’t know what I would have done without your knowledge, You’re truly gifted. I’ve been your student for 3 years now.
I miss class and come watch ur videos...then I sabawel while my mates r struggling but attended class😅
I'm making an A in Chem II because of you. Thanks!
Thank you for teaching me more physics than all three of my physics profs COMBINED!!!!!!!!!!!!!!!!!!!!!
bro is bringing back my love for chemistry. tysmmm
absolutely great with clear explanation it is so great to have you
You are idle example of
UA-cam's best
*Excellent video Sir.*
You are best teacher in my whole life. I am your very big fan. Salute sir.
My god, I am SO thankful for you. You are truly incredible. Is there anywhere I can donate?
*You deserve more views and subscribers.* I am sad now. 😩
You are very talented man !😎
Sfa Shaikh i m thinking the same bro....de deserve much more than he has..
Thank you thank you thank you. Your videos are extremely helpful and informative. You should be teaching at a University.
y'all are life savers!
Hope my exam over this goes well. I feel much more confident in it now.
I can’t say how much I appreciate your videos. Thanks sooo much I really mean that:)
You made chemistry very easy for me .. thank you sm 💯💯
You literally have been saving my life !!
Bro your better then my professor at explaining this. Thanks you very much!!!
Thank you very very much, I watched your video before reading my notes from my prof, now everything is clear and easy. Thanks again, appreciate your hard works!!!
I learn a lot from you! Thank you so much for putting so much effort and time into making these videos.
thank you for your video and giving the internet free education❤❤
Best chem tutor ever
*Best tutor ever
I like your method of teaching and crystal clear explanation. But one major demerits of your video is the very low volume of voice
13:20 I dont know wen it comes to electro chemistry whether or not my syllabus is different from what you are teaching here .I check my textbook and this what you're explaining ain't in there .
But overall you're the best 🔥🔥🤟
south africa learner?
@@heavenlyway108 Yess ...you see I go to a public school and we Do Caps I know private schools they do Cambridge or something idk what its called but we might have the same syllabus but theirs are more little more harder and different from what we do
You're amazing couldn't have done it without you
At 59:13 you did their sum wrong its supposed to be 1 volt 0.23+0.77=1
2:16 What if We plug in the battery to outlet and to my device at the same time..... it's (the battery) is receiving energy (electrolytic) and also supplying energy (galvanic).....how does this work ?
Mistake at 32:52, this is not a product-favoured system, Q is the initial concentration ratio. However, the positive standard cell potential is indicating that it is a product-favoured system since the reaction is spontaneous.
Am I correct?
58:14 it is 1 volt
yes I also got 1.0 V
This man is shaving my A levels
covering a whole topic in one vid thanks sir
you are saving my life with your videos thank you so much !!!!!!
Thx for being so good at explaining this. Gonna be taking my AP Chem exam later today!
Thanks....will be taking my chem 101later tomorrow
How was it
You have always made me to love chemistry. I like the content yo you deliver
The best teacher♥️
Thank you so much for breaking down the trends of the components!
Always a pleasure.
Thankyou!
Hey you! Long time no see!
Literally, have a final tomorrow. Wish me luck guys!
good luck
I LOVEE O CHEM TUTOR !!!
@59:00 how is the E =1,1V? Shouldn't that be 1,0 V? Because 0,77 + 0,23 = 1,0 V
31:59 into the video how do I get the answer 25080. 1.1v - 0.97 ÷ 0.0591 can't get the answer as he not say if you multiple by 2 or 10 or just 1.1v - 0.97. Help
What a great explanation!
Ur the best at everything
the best channel ever I will follow you always thank u for all
I got into harvard neuroscience because of u!
14:58 Why it's 212 Joules when you calculated -212267 J/mol?
Great stuff honestly
Hello, thank you so much for the video. In response to question 3 45:57, should it be 1776.61 hours?
no
Your videos are so helpful! Thank you so much for making them!
Thanks these videos are really helpful, I hope you have tutorials about Unit operations as well. Me and my batch mates are able to study solution manuals but it would be really helpful if there are tutorials or explanations as well ( especially Unit operations )
Anyone else studying for AP exams
BeyondLimits bcc cxxhxyjj g. Cj
Me 🙋
Nop, me studying for mcat
@John Wayne when are you writing? Im writing on 28th.
Cudos to you guys I'm still in Analytical Chemistry 😁
Your videos are amazing thank you.
SO many examples, thank you
On the last question what happened to "the one with the most positive cell potential will run as reduction" why was there are exception for cl2
Could you write what you say on the video because l dont speak English well
No
If you turn on captions by pressing on cc you’ll see it
Thank you so much for your service.
did anyone ever tell you that you're the best?!
thanks Midterm tmrw, wish me luck
All these ads about code when I know how to code lol also great videos
I wish I could give you all my coins but I’m wasting it paying for college.
You made this seven years ago before I even started A levels but here iam
How do you know the standard potentials? Most have more than one possible charge so how do we go about choosing the correct one?
how is the cathode less positive if its supposed to be more positive then the anode?
can you discuss regarding how Electro Chemistry apply in waste water treatment and Metal recover from Electronic Industry waste water with Copper and or lead particles in their waste water
Thank for your services sir
How does he know, the concentration for Zn^2+ and Cu^2+. That it is 0,01 and 10?? Please answer
cramming for my final
Am a pharmacist because of you my Herero
i thought E(cell) = E(cathode) - E(anode). why does he add it?
@ 58:50
Around 31 minutes he was talking about bringing the -1 from the denominator up to the numerator, and I was just wondering why doesn't the n also become negative?
thank you so much.............i think u should continue a communal service