Le Chatelier's Principle Part 1 | Reactions | Chemistry | FuseSchool
Вставка
- Опубліковано 2 чер 2013
- Le Chatelier's Principle Part 1 | Reactions | Chemistry | FuseSchool
What exactly is Le Chatelier's Principle? And why is it important to learn it to understand chemical reactions? Find out in this video!
Part 2 found here: • Le Chatelier's Princip...
JOIN US ON PATREON
/ fuseschool
SUBSCRIBE to the FuseSchool UA-cam channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
VISIT us at www.fuseschool.org, where all of our videos are carefully organised into topics and specific orders, and to see what else we have on offer. Comment, like and share with other learners. You can both ask and answer questions, and teachers will get back to you.
These videos can be used in a flipped classroom model or as a revision aid.
Find all of our Chemistry videos here: • CHEMISTRY
Find all of our Biology videos here: • BIOLOGY
Find all of our Physics videos here: • PHYSICS
Find all of our Maths videos here: • MATHS
Instagram: / fuseschool
Facebook: / fuseschool
Twitter: / fuseschool
Access a deeper Learning Experience in the FuseSchool platform and app: www.fuseschool.org
Follow us: / fuseschool
Befriend us: / fuseschool
This is an Open Educational Resource. If you would like to use the video, please contact us: info@fuseschool.org








WOAH, A GOOD MICROPHONE ON A CHEMISTRY VIDEO. MASHALLAH!!!!!!!!!!!!!!!!
me when i found the video 😭😭
these guys explain concepts in chemistry that i've always found confusing in a way that's so easy to follow, even for my dumb ass :)
YES THEYRE SO HELPFUL RIGHT?! EVEN MY POOPY BRAIN UNDERSTANDS THIS NOW
Love everything about this video, the choice of colors, how everything has a mad face, the simplicity , no background noise and the calm voice explaining it shortly, thank you!
THNAK YOU SO MUCH FOR THE POSITIVE FEEDBACK. WE APPRECIATE VIEWERS LIKE YOU
I watched other videos still didn't get it but when i saw the balance idea in this video I totally got it thanks!
will be out very soon - we are currently producing many videos per week - and 300 till September. We ll let you know here once it's out ;)
This video is probably the best one on the topic,straight to the point and spot on,THANK YOU
Thank you so much for the positive feedback. We appreciate you
Unbelievably helpful video .. Absolutely incredible .
So well explained, thank you!
Thank you so much!
I understand it better! God bless you.
You are so welcome. Thank you for the positive feedback. Stay connected with us on Instagram for daily posts. Be blessed :)
Fuse, you've restored equilibrium to my life with these lessons, thank you. xo
ew
cringe
@@nethernom cringe yourself
@@hachi7658 cringe you aswell
Thank u sm for this! This vid is so short and simplified but also very effective. I watched the other videos on yt but still couldn't understand the concept clearly. But now i do. THANK YOU AGAIN!
this is incredibly helpful! thanks
Super helpful thank you so much
easy to understand 😍
Wonderful explanation!!!
thank you so much you explained the concept extremely well❤❤
Thank you so much! I love it ^^
Very nicely explained.thnx a lot
Best chem channel!!
Thank you!!!
Great video. Tysm.
the reason why i have this feeling is that in reversible reactions due to temperature change the reaction produces its own heat in one direction & then uses it to go backwards
( Thank you for the awesome video )
Thank you so much!
Amazing 😍 its so simple the way u explain it
Thank you so much 😃
Thank you Fuse u have helped me understand what I need to know for my test.😀👍
So glad! Always happy to help 🙂
What a great method of teachind.🥰.
I understood all the points .Thanks so much 👏😊
Thank you too! Glad it was helpful!
nicely explain..thanks
Awesome saved me all guys subscription and like plzzz really helpful
helped a lot thank you!
thanks
Eisa, did you find part 2 in our chemistry playlist? It is already out =)
ツ
Very Informative. I understand now
Great! 😃
Thank you so much
This is the best video
You videos are just amazing,I can easily understand physical chemistry in your videos. Especially that equilibrium example was superrrr
Wow, thank you! Glad you liked the video.
GREATTTTTTTTTTT
Well initially in school I couldn ' t decipher this concept...but after watching this vid , le chatelier' s feels like a breeze . A big thankyou ! :)
That's great to hear - I'm glad we could help!
PERFECT
Wow.... Thank you for dumbing down this complicated topic with simple visuals
thanks, teach
U guys are so underrated.❤️
Thank you! Appreciate it!
Beautiful
thank you! this video was the most helpful video I found. Loved the animation btw!
Good.
For full syllabus of cbse chemistry, may also be referred below for value addition:-
World of chemistry class 11 and 12.
Life saver! Best explanation of Le Chatelier's principle... thank you, I hope I won't fail my test this Thursday!
+Patricia N'Khani Bungi That is great to hear!! Good luck in your test.
for 2 years i had been trying to understand the shifting of equilibrium
my teacher explained it to me over ten times and i watched many videos over 20 and 30 minutes then i come to this 4 minutes video and everything got clear
cant thank you enough
That is brilliant news! So happy we could help.
For pH indicators, is the stress coming from the pH of the solution? Like the liquid pH indicator is being put into a solution of a certain pH and because of that, the equilibrium is changed and then thats what gives the color to the indicator?
Really GOOD!!! Thanks! It was such a hard thing for me to understand from the book, but this video made it so attractive and arresting that i feel I donot have to look back to my books. Thank you once again. :)
the magic test-tube balancer reminds me of one friend I have. She's always balancing the atmosphere by setting people into place. like a moral police
OMG THANK YOU SO MUCH! I NEED TO PASS CHEM DURING SUMMER SCHOOL AND I HAVE A SERIOUS TEST TOMORROW AND NOBODY WOULD EXPLAIN(even the teacher) you helped me *SOOOO* much THANK YOU
this video is so helpful, thanks alotttt!
Thank you so much!
This was so well explained!
Keep it up! :D
😘😘😘😘😘😘😘😘😘😘😘awesome man
Thnxs so much
Most welcome!
helpful
Wow! Simply Wow! *.*
Wow! You're awesome Zebunnisa! Thank you for the kind comment!
can you tell me which program do you use to make this video?((( I want to make like that in my language(
Our designers use the adobe creative suite.
thank youuuu
Most welcome 💜
I can understand how pressure & temperature change & how they effect the reaction & force it back to the opposite direction to maintain equilibrium, but i don't really get it when it comes to concentration change!
a certain amount of A & B will react to produce a certain amount of C & D, how would the concentration of C & D increase! do we just add more of them ?
( Thank you in advance for any help you might be able to provide )
I also think ( plz correct me if I am wrong ) that reversible reaction ( backward reaction ) due to temperature change is more likely to occur right after the forward reaction is done, without any interference.
-
while on the other hand the backward reaction due to ( pressure & concentration ) would require something that causes the pressure change ( I dunno like doing the experiment on a top of a mountain - i know this sounds silly - ) or adding more substance to increase concentration!
Thank you so much :*)
No worries! 👍
good
👌
I was having problem in understanding the effect of pressure thing bt dis video explained it impressively...
Thanxxx
That's great to hear!!
omg thank u sooo much, this video has been really helpful!!!
You just helped me get at least 3 questions right on my test !! :)
I have a doubt. What inside the reaction cause the balance in equilibrium? What is it called?
love u bestie
OMFG I FINALLY UNDERSTAND THIS THANK YOU SO MUCH
Thanks!!!!! This was really helpful
Amazing, as usual!
I also don't understand why when volume increases equilibrium shifts to the side with more moles. They already are occupying more space as there are more moles. This means they have less volume. So why would equilibrium shift towards them? To further decrease the space?
I've wondered this myself but i think it's cause if you decrease volume it will cause a shift from stp so it would have to shift back, plus maybe something to do with K and the coefficients when calculating K
kkwan9928 thanks queen!
Still confused about the decrease of products. Surely you can't increase the products without decreasing reactants?
how to download this video?
Balanced, as all things should be...
I love you
true love, man
This is true love
Hi Ahmad,
Sorry for the delayed response - basically, adding C and D is a hypothetical situation - a stress that we are putting on to the system, and then seeing how it would respond according to Le Chatelier's principle :)
I hope this helps a bit, please let me know if you want to know more :)))
1:20 Shows knife and blackmailing it to go that side 😂😂
why dont u make all chapter videos and every part of chapter
Bom dia colegas
awesome vid!!!! extremely helpful!
i'm glad i found this video. thank you :)
So what does equilibrium on its own mean...
To make it into a Balanced state????
This video might help: ua-cam.com/video/wlD_ImYQAgQ/v-deo.html
If A + B are reacting to produce C + D, why there would there be a decrease in C + D in the first place?
❤ thanks. Viet Nam
Thank you for checking out our content. hope you stay subscribed with us.
I don’t get it why when the pressure is increased, the equilibrium shift to the side with fewer moles?
Thank you so, so much!
#Helena
possibly the best explaiantion on le chatalier principle!
just want to know which idiot disliked this?
on its way! :)
are they really a friend if they are filling your own hole?
It was helpful❤️
This video was favouring the conditions in exothermic right??!
helped alot short simple and straight :)
Short and sweet i love it
hi guys
i would love to translate this video to hindi how can i help!
It'd be amazingly helpful if you could do it through youtube's translations & transcriptions community contribution area: ua-cam.com/users/timedtext_cs_panel?tab=2&c=UCS3wWlfGUijnRIf745lRl2A
You submit the subtitle translations, and we'll accept them. Thank you!!!
Made easier in Kenya's 8.4.4
WHERE IS PART 2
I don't like that analogy. They seem to say that chemical equilibrium means equal quantities of each species on both sides, which is not necessarily true.
woow ! so simplified ,, loved it !
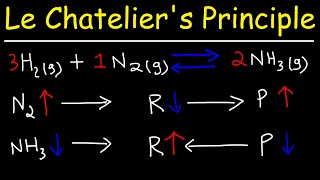
Let's say i have N2 + 3H2 --> 2NH3. I've got 43 mole N2, 152 mole H2 and 335 mole NH3. When I add 15 mole N2 to the system and await equilibrium, what will then happen to the ammount of mole of H2? Will it be more, less or the same?
the system will oppose the change.
Equilibrium will shift to the right to use up and reduce the N2 added.
So H2 gases will react with N2 to form more NH3.
amount of H2 decreases( to become less than 152) while amount of NH3 increases( more than 335).
Remember: the opposite of Addition is Substraction.
You adding more N2 will cause the equilibrium to reduce and use up the N2.
Hope that helps.