Alkenes & Alkynes Oxidation Reduction and Oxidative Cleavage
Вставка
- Опубліковано 14 гру 2024
- Leah4sci.com/redox presents: Oxidation, Oxidative Cleavage, and Reduction reactions for alkenes and alkynes.
Need help with orgo? Download my free guide '10 Secrets to Acing Organic Chemistry' HERE: leah4sci.com/or...
This is video 2 in the Organic Chemistry Oxidation/Reduction video series. Catch the entire series along with my redox practice quiz and cheat sheet on my website: leah4sci.com/redox
For more in-depth review including practice problems and explanations, check out my online membership site: studyhall.leah4...
For private online tutoring visit my website: leah4sci.com/or...
Finally, for questions and comments, find me on social media here:
Facebook: / leah4sci
Twitter: / leah4sci
Instagram: leah4sci
Pinterest: / leah4sci


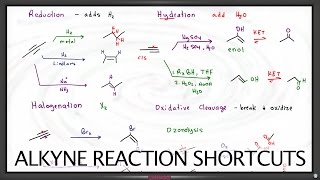






still a little confusing, but we are getting there. Thanks Leah! I think oxidation just isnt my thing haha. But like you said, if you see each pi bond as an oxygen that will be formed, it makes more sense.
Glad the video helped you understand the topic better! You are very much welcome!
This is short and precise. Good for revision. Thank you , Leah.
You're so welcome!
a level chemistry is so hard... being a science student is hard
Don't give up! keep pushing! :)
Come to india 😂😂😂😂 then u think being science student in their is so easy ..
@@akshayyadav6331 damn 😂😂
Being a pharmacy student is a bigger challenge 😢
2:58 so if addition of these 3 won't show oxidation, then these also won't show reduction too right?
Correct. There is no net effect on the oxidation state of the molecule by the addition of a water molecule, alcohol or hydrogen halide to an alkene.
8:03 when using KMnO4 to form CO2 the reaction condition is hot and acidic?
Yes, but I think I'm 4 years late 😅
Hot and basic, then you acidify in the next step
Thank you for the amazing videos. It's really helpful for my MCAT preparation🤩
You're very welcome!
You drew the syn addition as anti addition
At which specific point in the video?
3:56 isnt that is a trans diol if u rotate the bond
If we considered the rotation of sigma bonds, then no diol would every be considered cis or trans. In saying this is a syn diol, I am trying to communicate that the alcohol groups are added to the same side of the alkene. It’s more about the mechanism of the addition, rather than the final structure itself.
Thanks for the step by step explanation
You're so welcome!
I have a question 🤧... I've watched the video but kinda failed to apply the concept.. I don't know where I'm missing it
Reacting octene(C8 H16) with KMnO4 under hot basic conditions giving propanoic acid and pentanoic acid.. but according to your video, reacting an alkene with KMnO4 should give an alkene and carboxylic acid
In my example for oxidative cleavage using KMnO4 in hot basic conditions, we had an alkene that was di-substituted on one side of the pi bond. That meant one side, when cleaved, was unable to oxidize any further than a ketone. In your example, if you're cleaving 1-octene, neither side is di-substituted which leaves room for further oxidation.
Thanks so much your video really helped me.. I don't know what I would have done without you:(
You're welcome!
Glad I could help!
Upload a video on kmno4 mediated oxidation of alkenes with mechanism please mam
For help with questions like this and more, I recommend joining the organic chemistry study hall. Details: leah4sci.com/join or contact me through my website leah4sci.com/contact/
Thank you!! You're very good at explaining!!
You're so very welcome!
how does cl and H cancel out?
Thanks for asking! Halogens, just like oxygen, are highly electronegative and considered to be strong oxidizing agents. Therefore, in a hydrohalogenation reaction, one carbon of the pi bond is oxidized by the addition of a bond to a halogen while the other carbon is reduced by the addition of a bond to hydrogen. The oxidation and reduction cancels out and does NOT affect the overall oxidation state of the molecule.
Helps a lot, thanks!
You're welcome!
oh my gosh that was amazinggggg
So glad you enjoyed it!
thank you so much
You're so welcome!
Love u your teaching madam
Thanks a lot!
thank you
You're welcome
well said
thanks!
nice video
Thanks!
Thank you🙌👍.
You're so welcome!
thank you mam
You are welcome!
thank you very very clear explanation and also you have the same voice as selena gomez hahaha keep going ;)
I take that as a compliment, thanks! And you're very welcome!
When your exam is in half an hour
How did your exam turn out?
Very good! I'm pretty confident in the rest of the topics, this is the only one I specifically struggle with and your video really helped. Thank you so much!
Every Good
glad you enjoyed it!
you are amazing
Thanks!
12:23
Did you have a question about this?
Oh no I just noted down the time stamp so that I could go back to it if I forgot how the reaction worked. Thankyou for asking! @@Leah4sci
Excelente! :)
thank you!
Bokka
?
for jee aspirants its a joke
Not sure what you mean
thank you mam
You're welcome!
thanks 😊
You're welcome!