Step by Step Density Practice Problems to Help You Pass Chemistry
Вставка
- Опубліковано 29 сер 2017
- Learn how to use density as a conversion factor and how to find density. This video explains what the proper units are for density and how to get the correct answer every time! Together we practice all types of density questions step by step to avoid any surprises on your exam.
📗 FREE CHEMISTRY SURVIVAL GUIDE
melissa.help/freechemguide
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
chemmunity.info/youtube
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
melissamaribel.com/
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
melissa.help/thermonotes
📗 Acids and Bases Guide
melissa.help/acidbase1notes
📘 Naming Compounds and Acids Guide
melissa.help/namingnotes
📙 Dimensional Analysis, Significant Figures, and Density Guide
melissa.help/sigfignotes
📕 Gas Laws Guide
melissa.help/gaslawsnotes
📗 Stoichiometry Guide
melissa.help/stoichnotes
📘 Redox Reactions Guide
melissa.help/redoxnotes
📙 Molarity Guide
melissa.help/molaritynotes
📕 Limiting Reactants Guide
melissa.help/limreactnotes
📗 Lewis Structures Guide
melissa.help/lewisnotes
📘 Kinetics Guide
melissa.help/kineticsnotes
📙 Titrations Guide
melissa.help/titrations
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
melissa.help/matterguide
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
melissa.help/cheggstudy
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
melissa.help/cheggbooks
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!
Density practice problems with step by step answers:
bit.ly/densitychempractice
In-depth "how-to guide" on density:
bit.ly/densityhowto
TIMESTAMPS
0:34 Density
0:51 Density as a Conversion Factor Example
2:45 Dimensional Analysis and Density Example
5:41 Finding Density Example
7:08 Practice problems
___________________________________________________________________
Music: Baila Mi Cumbia - Jimmy Fontanez, Media Right Productions • Baila Mi Cumbia - Jimm...
___________________________________________________________________
Practice problems in the video:
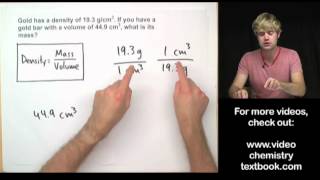
What is the density in g/cm^3 of an unknown solution containing 2.3kg and 8.0L?
Answer: 0.29 g/cm^3
If ethanol has a density of 0.789g/mL and a mass of 36.4g. Find the volume in mL.
Answer: 46.1 mL
The density of a solution is 0.791g/cm^3. What is the volume in cubic inches of 2.7lb of the solution?
Answer: 95in^3








Anybody else overwhelmed??? 🤨
All the time! What is overwhelming?
@@melissamaribel everything
who isnt? chemistry is not easy!
I have a question. Did you just memorize all the conversions and do you have any tips on memorizations for them?
I have been getting F's all this semester and let me tell yu this was a piece of art. This video helped a lot and I have bumped my grade all the way up to a D-!!!!!!!!!!!! I got an A++ on the assignment.
Thank you So SO SO SO much!! LOVE your vids keep making them!!
You're very welcome Kayley!! SO happy you are improving 😊
This video is a life saver! I watched at least three other videos on density and this video was the one that I was able to understand. I love the way you broke down each problem.
Step by step yet so simple. This channel is outstanding had to watch some videos for my chemistry class. This is so simple to understand. Perfect job, now I understand this a lot. Thank you!
Thank you so much for this video. I have to pass Chemistry in a matter of weeks in order to be able to start grad school on time. Praying for a miracle but your channel is definitely helping me. I will be referencing all of your resources!
I am so happy I discovered this channel! Melissa Maribel, you have helped me so much get through assignments a little bit easier. Can you do a video on the relation between molarity and density, or percent yield? Thank you!
You’re saving my life for my exam!!! Thanks for being so kind and helpful ❤️❤️❤️
Thank you so much! I learned more from you in 1 video than from my teacher in 1 week :)
thank you, this was soo helpful! love your little dances during the problems at the end
i love step by step vids thank so much . i been trying look for something like this
thank you some much. you helped me understand better and even gave me information i missed during the lecture by the teacher.
I love you. You're literally a life savor - THANK YOU
OMG so glad I just ran across this, so helpful.
Your videos help me so much!
Thank you so much. You placed the final piece for me
THANK YOU QUEEN UGH I WAS SO STUCK BUT U JUST HELPED ME😭😭
Happy to help 👸🏻
Thank you, your tutorials have been helpful. Recommending you to my classmates. :D We're all in this together.
Thank God without your videos i don't know if i could pass my chemistry class .... phewwwww 😅 thank you !!! ❤️
This has just saved me so many tears. Thank you.
Hey, great video as usual! When you're doing all the conversions you don't round them to sig figs correct? For the last example I keep getting 94.48272009 in^3 and rounding to two sig figs would get me 94, I am assuming I am a few decimal numbers off because of sig figs or when I cubed 2.54cm?
This is great!!
Literally didn't understand my homework and no lie almost dropped my chemistry class because I seriously figured I need to perfect algebra ....I came here and I feel wayyyyy better.
I am really sad you aren't my teacher lol.
Yay! I got all the practice problems correct! The last one was tricky because of all the conversion factors. I was unaware when I come across something like 75g/mL that I can make that into a conversion factor. This is so new to me. I always got stuck when I came across something like that. I also was unaware you can square or cube a whole section in a conversion factor. My professor kept doing this last semester and I just didn't understand. I'm like where did that come from? How can you do that. I was completely lost. He just kept responding oh you will get it sooner or later. Last semester was online so it was very difficult for me. I passed the class with a "C". That wasn't the grade I wanted. I will be retaking Chemistry(I) in person this time. I want to get a better grade and retaking this class will help me better understand Chemistry (II).
I don't understand Melissa every time I do the practice problems at the end of your videos I am getting everything correct and I feel like a rock star but when its with school its seems more difficult and the way the problems are written are confusing. I end up failing or barely passing. Your practice problems are straight forward and easy for me to understand.
How do you know to use cm3 to get to grams?
Thank you I was so lost at school now I know what I'm doing
You're welcome Ana! So happy to hear it's making sense now 😊
i feel likei wiil benefit from keep up with the good work love you thanks
I LOVE YOU THANK YOU QUEEN 👑
How did you get 0.008 on the last one?
Do you happen to have a place I can go to see the steps for the practice problems at the end?
Imma pass chem thanks, I like how your organized
just got done watching and this was quite helpful however i still need the help. the "in deth how to guide on density" is not working. when i click on it it says it does not exist.
How did you come up with the 2.54 at the end of the problem that needs to be cubed.
Do you have every single conversion factor memorized?
how do you find the conversion factors? how do you know you are to convert lbs to kilograms ?
God Bless you Melissa! This is really REALLY helpful!
Thank you so much :)
Hey where did you get the 10^3g from?
Following same question, how would you know the all conversion unit when it's not given all the time?
I understand how to find density but I can’t figure out the volume. My teach just gave us a question that said “you have a 250 mL graduated cylinder containing some water. You drop three marbles with total mass of 95.2g into water. What is the average density of a marble?” In order to find the volume I need the initial water amount and then I’ll be able to solve for the volume of the three marbles no? I’m confused on this part I hope someone sera’s this. This is suppose to be review of Honors chem to prepare for AP chem but if I can’t do this then idk how ima do In the class.
this lady saved me in chem
Why can’t you be my professor!! This was sooooo helpful!
Aww thank you!
I’m a little confused on how you set up the table for the second problem
Hi, I did the last practice problem exactly how you showed in the video and I got 59 inches cubed and I was wondering if that's what you meant by 95 (just a typo) or if it was something I did wrong?
Hey Kimberly so double check your set up for this question because it should give you 95 inches cubed. Here is what the set up should be:
2.7 lb x (1kg/2.2 lb) x (10^3 g/ 1 kg) x (1 cm^3 /0.791 g) x ( 1 in / 2.54 cm)^3
Make sure to cube the last conversion factor so you'll have this:
2.7 lb x (1kg/2.2 lb) x (10^3 g/ 1 kg) x (1 cm^3 /0.791 g) x ( 1 in^3 / 16.387 cm^3 )
ok , but i need the song name to that little cumbia beat . i was celebrating the correct answers with you !!!
I keep getting 215.3 in^3 and not 21.53 in^3.. I'm confused. What am I doing wrong?
Instead it’s units: g/c^3 .. right?
I'm trying to find a video that finds volume because my teacher explained it once in a non word problem and never went over the density part of the review guide.
Excuse me Melissa, @4 minutes and 56 seconds in to the video I keep getting 0.2152629898 on my calculator. It makes me really confused how you got 21.53.
(I understand sig figs and rounding so I'm not concerned about all the extra numbers behind my decimal point...I'll get that cleared up when I apply sig figs and rounding...I'm just confused why your decimal point is behind the 21 and not before...thanks
I love you I love how you have a cute UA-camr setup but it’s chemistry ! So fab.
Thank you so much Emily!! This just made my day because this is exactly what I'm trying to do, give Chemistry a little makeover 💁♀️
Chemistry with Melissa Maribel you’re so cute! Watching your videos currently & taking notes. Do you provide any online one on one tutoring?
Thank you Emily!I just opened up a few spots for tutoring, you can check times and sign up here: bit.ly/melissatutor
for the density problem where did the 10^-3 come from?
so how do we know 1mg is =10^-3? I get the set up I guess now my issue is knowing for future problems how 1mg=10^-3
Where did you find the conversion factor of 4.51g = 1cm^3? I keep on finding on the web 1cm^3=1g.
i know this comment is old so you probably got the answer already but it’s from the density provided, 4.51g/cm3
@@horsenberg6175 Would u mind answering my question on why we don't transform our final answers to scientific notation when solving density problems?
hello, I suck first of all and also I m soooo lost whats that cm3 where does it come from every exercise I have it's like that, and I suck at math now at this. im doing online classes, so it's very hard. i know m is for milliliters but the 3 ???? it's killing me especially when I see a negative 13, cm-14 -10. what are those
I love ur vids but my problem is how do you know you you need to go to mL to cm to g?? I don’t understand how that happens why not ml to nm?? Like how do you know its to cm??
It’s because there is no conversation factor that allows you to jump from mL to nm. But there is a conversation factor for ml to cm. Which is the 1 ml = 1 cm^3. From there you can use the density to help you go to grams. If this isn’t making sense I recommend watching these two videos first they will help you better understand the setup. ua-cam.com/video/7voNNBbMcxE/v-deo.html.
ua-cam.com/video/HH-jCJKaPUs/v-deo.html
Chemistry with Melissa Maribel thank you so much!
Why don't you transform your final answer to scientific notation? I've been taught scientific notation a lot with dimensional analysis but now I notice that in density no one uses scientific notation anymore...
I wish you showed the practice problems steps
I'm having problems finding the density of a metal cylinder in water
hi, I'm still having trouble with conversions! for example, how did you memorize that 1 mL = 1cm^3???
Keep a flashcard nearby with all the unit conversions. With practice and referencing often , you will remember it. 1ml=cm^3
I like very much 😍😍😍
Can you do a video on solubility curves? :)
Melissa Maribel mkay thank you:) that’s the new unit now starting to get harder and harder
Melissa Maribel how can I join?
Am I supposed to already know the conversion factors? :(
thats what my question is too
Diego yes, if you are looking at this video, you should be comfortable with conversion factors. If not, Melissa has a tutorial on that subject. I suggest you watch that one first.
@@jacksipe9573 Thanks for the heads up!
Your links to the practice density problems are broken. Can you reupload them?
Thanks for letting me know. Here you go bit.ly/densitychempractice
How come another tutorial teacher says you should leave the answer in 3 significant numbers? Please respond
His name is tyler dewitt
The number of significant figures depends on the value you are initially given. For the first example at 1:03 the question gave us two different numbers, 25.0 and 0.702 both values have 3 sig figs. Therefore your answer will be rounded to 3 sig figs. If we instead have 25 and 0.702 we would round to the lowest amount of sig figs between these given values. 25 would have 2 sig figs and 0.702 has 3 sig figs so our final answer would only have 2 sig figs.
Chemistry with Melissa Maribel Thank you for the explanation 🙏
thank you you save me there fore I am 6 class student and our study is so tuff if my english is wrondg so tell me I am from pakistan
Hi!! I was wondering if you are still tutoring?? Please let me know! Thanks
Hi Rita, I'm no longer tutoring but for the future I will be creating chemistry courses on chemmunity.com. This semester I'm starting with OChem 1, so if you have to take that in the future, my course will be available for when you need it.
@@melissamaribel awwww...ok thanks anyway 😊 I'm currently taking chemistry 092 for nursing
Why are we converting lbs to kg, why not just convert lbs to grams?
You can do that but for the sake of using all the conversion factors and making the problem look simpler, she used kilograms. If you find an easier way to do it like skipping the kilograms and directly converting to grams, you can.
I need help cause I'm confused Maribel
What isn’t making sense?
i dont understand
Hey Trevor, what isn’t making sense?
on the first do it alone, why was 287500 turned into . 29 without using any scientific notation. we cant turn large numbers into small without it.
@@melissamaribel ?
@@miapurnell6142 1) 2.3kg = 2300 grams
2) 8.0L = 8000 mL
3) 1 mL = 1cm^3 which means that 8000 mL = 8000 cm^3
4) 2300 grams / 8000 cm^3 = 0.2875 ----> Round your answer to the least amount of sig figs, which is 2. Thus,
0.29 g/cm^3 is your answer
Me either the weird thing is I was taught this in 8th grade I got use to it, and I'm sure I was pretty good because I passed with a b... But then it just came back and said "boo I'm here to haunt you!! " In the 10th grade and I forgot how to do literally everything... So I came here to review my knowledge and I'm still lost I believe because I was taught this differently
I am going to fail this class
While I did the math I got 21.43 instead of 21.53... what am I doing wrong??
Melissa Maribel I luv u so much
lord help me......please
Amen 🙏
For the density of titanium problem, couldn't you go from lbs to grams directly using 454g=1 lb so you don't have to use so many conversion factors? When I do it this way I get 21.54824624