Sigma and Pi Bonds: Hybridization Explained!
Вставка
- Опубліковано 6 лют 2025
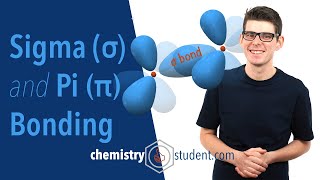
- Sigma bonds are the FIRST bonds to be made between two atoms. They are made from hybridized orbitals.
Pi bonds are the SECOND and THIRD bonds to be made. They are made from leftover "p" orbitals.
Check me out: www.chemistnate...






I've spent literally HOURS trying to understand this and my university lecturer couldn't even make me understand it, but in 8 minutes and 2 seconds I've just understood, thank you!!!
rerally ...only from ur comment ...i think to watch ...dekhten h kitna sahi h madam
i love you
Can u explain why is it sp3 or sp2...i know its a sigma bond but ??
Nicole C me too
I know that this is 3 weeks late, but I just found the video studying for my chemistry exam, and decided to answer.
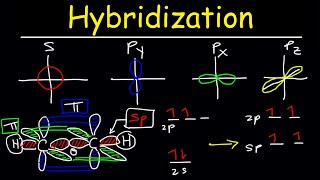
We can determine the hybridization state by the steric number, which can be found by adding together the # of sigma bonds and the # of lone pairs. If the steric number is 4, then the hybridization state is sp3. If the steric number is 3, then the hybridization state is sp2. Lastly, if the steric number is 2, then the hybridization state is sp.
For example, a carbon with four single-bonds will be sp3 because steric # is 4 + 0 = 4
A carbon with two single bonds and a double bond will be sp2 because steric # is 3 + 0 = 3
Another example is when an element has three bonds and a single lone pair of electrons. sp3 because 3+1= 4
I hope that this explanation helps clear things up a bit.
I just wanted to take a second to sincerely thank you for these videos. 12 years later after this was posted, this is helping me with an upcoming chemistry exam. You explain everything so simply and your videos are you have a great personality that makes learning fun with moments of laughter.
This video was made 8 years ago and it is STILL helping people understand! I spent hours trying to understand what you were able to explain in 8 minutes. Great video! Truly grateful. Thank you!
I am deaf and his hands explained everything. Thank you man!
really
really ???
omg really?? I didn't knew that..
and I even thought his hand movements were annoying since I couldn't concentrate..but the explaination was good.thank you😊
it's a joke guys, you need to take a lecture on gullibility.
Caption *sigh*
I almost cried for how good you explained this. Thank you very much.
Me too
Seriously. Best video!!
You said it! Cheers, Nate!
U must watch KGF 1 and KGF2 movie
U must watch KGF 1 and KGF2 movie
Why are teachers and textbooks so uselessly dense? People need to be more clear like this guy.
+Minka Kelly calm down
+Na Mirae uhhh
+Minka Kelly Teachers and textbooks weren't built to teach you a concept in 8 minutes. I love this UA-camr because he simplifies things when I need to cram, but it took me about 3 hours of class time and another hour outside of class to learn about hybridization in detail.
And don't hate on teachers. They go through years of work just to be a teacher, and some of them actually WANT to be good teachers. Give them feedback and help them be better!
+rickybobbychuva Oh man...if only my AP Chem teacher was responsive to feedback. Every time we tell her she needs to explain something in more detail, she just says, "You need to know it for the test." Grrr....
Don MMA Yikes, I've been there bruh. Some teachers really care, but there are some teachers that make me wonder why they became teachers in the first place. xP
If only profs made it this straightforward, rather than try and guide first-year students into the very heart of the theory itself, they might do better. Thanks for simplifying it Nate.
+YesNoMaybeSometimes Maybe if people payed attention to the teacher and can learn the small details this guy left out. Professors teach you everything. It just shows how stupid someone is if they have to have it simplified to a 3rd grade level.
Countless videos, lectures, and google searches and your is the only video that helped.
THANK YOU I've been watching videos on hybridization for an hour and none of them were making sense! You are the only one who explained it in a way I could understand!
Haha yes, out of all videos, it was this video that made me understand!
The name's bond, sigma bond.
A V 😂😂😂😂😂😂😂😂😂😂😂😂😂😂😂😂omw
ur cool
That moment when you make an epic chemistry joke, but still get no "REACTIONS"...
Ba dum tss
Ohmg such a nerd
Nice to meet you. I'm Bond, Pi Bond.
Dude... if we had more teachers like this we would be done with classes much sooner. You explained it so good. Thank You
I have searched and searched for people anywhere and everywhere on the internet to explain this stuff and no one has come close to doing it as well as you. You explain it so energetically and in an easy to learn manner!!!
That was probably the best, simplified and precise explanation I could ever have on all the sigma and pi bond stuff. I finally got it now.Thank you SO MUCH. You knew how to explain it clean and clear!
WOW, you took 8 min and 2 seconds to get me to understand this but my teachers at school took FOREVER to explain this to me. You are a GENIUS!
This guy is amazing ! my professor sucks at explaining sigma and pi bonds thank you
+GoogOOL MaNgooli Dude I got one who can't even speak the language (portugal here), all he does is point, say something you struggle to understand a letter, and say "ok?" and moves on.
This really helps a lot, hopefully I pass the exam and escape from his grasp.
my ochem pro did not even explained it
sir i cant even begin to tell you how thankful i am. i watched everything on youtube but got nothing useful, this was pure gold. thanks
U must watch KGF 1 and KGF2 movie
Your explanation is really detailed. Somehow my school lecturer does not seem to explain in such a way, expecting everyone is smart enough to understand through only a brief explanation. I understand alot more by going through your video. Thank you, please keep making more chemistry videos!
U must watch KGF 1 and KGF2 movie
Basically I am a mathematics teacher but still it's good to watch your videos
hand movemnet killed me haha
rip
hahaha same cant focus on the video due to continous laughing xD
Its that chemistry jutsu
Lmao
superpe
😂
Honestly thank you. Textbooks and lectures are useless. I took 10 secs of your video to make me understand. Our educational system needs more people like you. well done
very clear! it took you 30 seconds to explain something that took my professor 1 hour to explain. LOL.
thank you!
Bless you, seriously. I had no earthly clue what pi or sigma bonds were and my university lecture was doing nothing to help me. I owe you my life.
You sir are a modern day hero. Your students are lucky to have a teacher like you. Many thanks!
The top comments are from like 6 years ago. I'm here in 2021 and you still make more sense then my university lecturers. Thank you!
What university?
Sigma and Pi bonding theory has been the same for years, so ...
Why not they just add those two sentences : "Sigma bonds are the FIRST bonds to be made between two atoms. They are made from hybridized orbitals.
Pi bonds are the SECOND and THIRD bonds to be made. They are made from leftover "p" orbitals." in text book.
He said that, first bond is always sigma at 0:19
Nina Duran
Alan is questioning why the textbooks can't make it this simple.
YesNoMaybeSometimes oh I see, we have it in our books so.
Nina Duran you may have been lucky to buy some nice one. i have got three and none of them has it #dontgetbitchyaboutit
because its oversimplization of the concept... if u dont understand the basis of why is it .... once u get to hardcore stuff this trick will fall apart and the actuall understand will come to rescue
Man… this guy just explained what I tried to learn in 2 years in 8 minutes….thank you sir!
THANK YOU SO MUCH! I have chemistry test tomorrow and my sir hasn't made me understand a thing about hybridisation! Thank you very very much! Helped me alot.
Five years later and I'm in the exact same situation as you. How are you doing now? How was uni life?
Sigma and Pi Bonds: ua-cam.com/video/Xr3uOwUzA8Q/v-deo.html😁🦜🐦🐧🌅🌄🚃🏙🎢🚋🚞🚝🌇🏞🏝🏜🏖🏕🗻🌋🏔🏫🏘🛖
This helped me so much, thank you. It's crazy how sometimes a simple definition in the beginning helps make everything so much more clear.
thank you so much for this video i learned more in 5 minutes watching this video then the hour i spent rereading and trying to understand my text! your a true lifesaver
After so many years of chemistry classes, I have finally found what I want. You are awesome, Nate !!!
U must watch KGF2 movie
Nobody never can explained s and pi bonds so crystal clear and easy way! Thank you very much!
Wow, this video made the hybridization concept make so much more sense! Using multiple videos really helps with chemistry. Thanks for making this!
the ONLY video on youtube that explains hybridization so effortlessly!! thank you so much!!!
Legend at work!
Won't be able to answer those complex high-school questions without the help of this. Teachers told me about the sigma and pi bonds, what hybridization is and all but they never told me a simple fucking thing which is - how they ACTUALLY KNOW carbon hybridizes in different compounds the way it does. Thanks! :)
My professor was so excited over these bonds, and it makes total sense in his head, but I had him explain it 2x and still was like whaaa? I screamed OH!!! THANK YOU NATE!! I have a feeling you are going to get me through orgo this semester.
tommorrow is my exam, i had being trying to understand this concept for about a month but wasn't successful. You saved my life man. Kudoos!!!!!!!!111
Chemistnate is not only funny but also super helpful. He is truly a miracle to my chem grade and his hilariousness makes me keep watching without getting bored.
Lol your hands are a bit distracting BUT I can say this was one of the best explanation of the sigma/pi bonds I've ever heard
7 years later and high school Ap chem teachers aren’t explaining this any easier. Thanks for the lesson man, it helped me far more than the teachers lecture!
thanks, you just helped out a dazed and confused 1st year college kid
Sleeping Wind Spirit Haha now Im the confused 1st year college kid
This is in college? I learned this in grade 9
@@mirumanzi and
@@mirumanzi prob u got crammed in some asian education system haha
how r u doing now sleeping wind spirit
I spent hours searching for a video to explain what this main explained in the first 4 minutes..... I cannot thank whoever made this video enough!!!! Honors Gen Chem 1 test Monday and i neeed to do well >.
God bless you dude, have an exam in 24 hours . you made this a lot easier for me
+26847890 Same here! Exam tomorrow, and I'm staying up late to study! This guy probably saved my ass!
Every UA-cam video out there sucked. They couldn't make me understand no matter how they explained it. But this video is great. This video is super-clear, the clearest of all videos. Out of five videos I have watched, I still had no hope in understanding hybrid orbitals until I have watched this one.
Thanks chemistNATE!
"this is a chemical bond that i just invented, i don't even know the name of it" I DIED
For two weeks in class I couldn't understand this ,but in 8 minutes and 2 seconds finally got it ..this was helpful unlike those useless textbooks and lectures
god bless this video! you dont know how much it helped me. im now 100% confident im gonna pass my exam
why cant professors be this clear when you pay them to learn this stuff, I've literally been lost on sigma/ pi bonds for the past 3 weeks and this 8 minute video removed all the ambiguity i was facing.
when you finally get it
"CHILLS COME DOWN THE SPINE"
Happened to me tho.
very usefull u just saved my life today i hace chemistry exam. i understood the topic with ur 8 min video than spending 4 days with my professor
Is this guy doing sign language during the lecture?
+Brittany Bettingen gang sign
+Brittany Bettingen lmaooo! now i can't focus
Naruto techniques
This was the first thing that came to my mind when I started this video. XD
Nate you ATE with this explanation. Ive been watching a couple videos on your channel, and I love how simple you explain stuff; you just go straight to the point! Thank you!! Im about to have my first orgo midterm in 3 hours and im more confident
He sounds like Deadpool, I seriously can’t stop thinking about this. 😂
This video is so helpful. I found it when I was first learning chemistry my sophomore year in high school. Now I'm a sophomore in college studying chemical engineering and anytime we talk about bonds in my classes I rewatch this video for review
My AP exam is tomorrow, this video saves my butt Thanks!
Same, good luck man.
+GoldenGreene you too!!!!,Get a 5
easiest and most straight to the point explanation i have ever seen or heard. thank u
What an awesome explanation! Clear and concise
Im from brazil, i was having problems to find good explanations about that in my language and you did a great job. Thank you very much, you got a new subscriber.
Sigma bond grindset
Finally got this. Took a few hours and watching and reading over and over through many different material, but this is the video that finally got me to understand. Thank you.
you're a living sigma grindset
This video was helpful.
It took me 1.5 years to understand hybridisation, but this finally helped me.
stop reading comments. listen to the video
Wtf... Then why are u writing and not listening
Hahahaha
bruh, you can literally use that comment on gaming vids and all, but im pretty sure everyone here came to learn so i dont think it really works
hahahhahahahq u got me
You know what 😭😭 I have spent 8 videos and 2 tutorials just to understand this chapter but I can't give up. You are my heroess😭😭 I really can understand all this chapter through your videos 😭😭😭 I'm not lying this is true I have spent 8 videos but still can't understand, but you make my day.TQQQ SO MUCHH
thank you!! helped me lots last year, and helped me again now.
Your explanations is waaaay better than my teacher. I'm not even an english native but i got it ! Thanks !
THIS SAVED MY LIFE THANK U
Your help has been massive
That rehearsed hand movements tho lol
i watched for like 50 sec on pi bond and it clicked!! unlike watching a lecture, and other yr videos! Thank u
Finally I understand this. Now I just need to remember a bunch of other crap and I'll be ready for mid-terms.
i have been staring at my book for 2-3 days and procrastinating every time i see the phrase sigma or pi bonds. thanks to you i realize that this is quite an easy concept to learn. hopefully you have a video out explaining molecular orbital theory since that's the next beast i intend on slaying.
My life is now complete
This video made so much sense! You taught me so much faster and clearer than my past lectures. Thank you so much!!
THANK. YOU.
Dude I took chem I and II a year apart from each other at one university then took organic chem I at a different university a year later and ALL of their ways of describing were different. I was so lost during organic and my mind couldn't fully grasp the term sp3 because no one had fully explained it to me and my education in chemistry was spread out over so much time that I forgot a lot of things. So the moment you drew out the electron configuration to explain sp3 I literally wanted to slap myself because of how SIMPLE it is. I can't believe I didn't piece it together sooner, thank you for the help
The hand gestures definitely made the video
OMG THANK YOU!!!! I have an ap chem test tomorrow morning and this has saved my life.
I feel dumber knowing that almost everyone understood this nonsense.
This is not nonsense, if these bonds and intermollecular forces didn't exist; you my friend woun't exist.
I know this is so hard
Jorge Cardona did you get your degree?
Man rly did u drop out haha
same here....
Thank you for explaining hybridization. I never understood how it worked until you explained sigma and Pi bonds.
Shout out to everyone taking the ap chem test tomorrow! We're all gonna fail! I mean get fives lol
U must watch KGF 1 and KGF2 movie
you are the only teacher who can make me understand chemistry, you explain in fast and simple
you heard it folks, aint no fictions
"Ain't no fictions." I was irritated by the constant hand gestures at first but glad I stuck through this. His explanation was clear and he was endearing :).
Sigma rule 12: bond with atoms
This was the best explanation ever
Thank you very much Sir
This is amazing...
I was totally confused after reading the book about hybridization but after watching this video it all makes perfect sense! Thanks for making it
He explained 5% of the full concept of HYBRIDIZATION..
Where is sp3d.. sp3d2 etc?? there are lots of things to tell about "What is Hybridization"
These are basics that many high school/ college students study. This is exactly what I need to know for my A level syllabus when it comes to hybridisation. There is a sea of videos out there, go explore.
xx
Sanvir Hasnat 'Channel Echank' watch Richard thornley channel,it explains everything about hybridization.
What kind of uni do you go to lol we need to learn sp3 etc.
hmm...the vid is about pi and sigma bond.Not about hybridization
CandyQueen which board are u in?...I am from CIE board lol
That really helped out even 7 years later! Thank you man!
I'm still wondering why the fuck professors don't explain ANYTHING like this. WHY.
Lecturing is totally different from tutoring. Lecturing introduces new concepts. Tutoring is helping with processing information. For most people, receiving new concepts is more difficult than processing old information.
That is why the first day on campus is stressful. It is not because people at universities are bad at giving directions, it is because students are having a new experience. The same goes with lecturing and tutoring.
This just cleared up something that my professor and my Supplemental Instructor could not explain to me clearly! After watching this video everything makes so much sense!
anybody else here pulling an all nighter before the exam?
U must watch KGF 1 and KGF2 movie
that's really amazing man ! ! !
I've my chemistry examination tomorrow and i got everything which my teacher couldn't even explain . . .
hats off to you man ! ! !
Fucking blessed video thanks man!
U must watch KGF 1 and KGF2 movie
I've been struggling for an hour to find a useful video , and then I did that ! Thanks mate !!❤😍
THANK YOU SO MUCH
This was the first video I watched where I understood hybridization! Thanks!
Very helpful thanks but shouldn't the pi --- 2p actually be 2p^2 since we have 2 pi bonds? at: 6:42 ??
THAT HAND DEMONSTRATION IS LEGIT.
AND VERY WELL EXPLAINED.... U DESERVE MORE REACH........