Hybridization of Atomic Orbitals - Sigma & Pi Bonds - Sp Sp2 Sp3
Вставка
- Опубліковано 21 лис 2024
- This organic chemistry video tutorial explains the hybridization of atomic orbitals. It discusses how to determine the number of sigma and pi bonds in a molecule as well as determining if a carbon is sp, sp2, or sp3 hybridized. The full version of this video contains plenty of examples and practice problems.
Access The Full 36 Minute Video:
/ mathsciencetutor
Direct Link to The Full Video:
bit.ly/3iv1Zic
Hybridization - Free Formula Sheet:
bit.ly/3YgxHkf
__________________________________
Organic Chemistry PDF Worksheets:
www.video-tuto...
Join The UA-cam Membership Program:
bit.ly/46xaQTR
Full 36 Minute Video on UA-cam:
• Hybridization of Atomi...


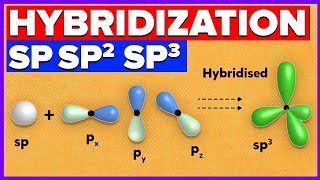





Hybridization - Free Formula Sheet: bit.ly/3YgxHkf
Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Access The Full 36 Minute Video: www.patreon.com/MathScienceTutor
Direct Link to The Full Video: bit.ly/3iv1Zic
Bro bless u
Just a quick note that this guy is literally better than all my chemistry professors. keep up the good work
facts
This guy has saved me in Ap Chem and in colleges Chem on too many occasions lol
you learn this in college??? bro this is junior year chemistry
I totally agree 👍 😊😊😊
@@pranhav this is general chemistry information, which you usually take in your first semester if you're majoring in something chemistry related
I think this is the only person on UA-cam, even on the entire internet without ANY haters
how could anyone hate on this man?
i do because he doesn’t upload the full damn video
@@ttbetterthatyt8468that's because he has a membership program where he uploads the full videos. I don't know why u aren't grateful for this guy, he has almost 3k videos for you guys to watch for free which u can't see anyone else doing
I hate him because he know chemistry and I don't
Says who?
This guy is literally a genius..He knows everything about chemistry, Physics and Maths..May God bless you for saving me all the time
I love that everything is slowly and logically explained, because although I‘m good at chemistry and I like it, sometimes the teacher’s pace is a little too fast. A lot of examples and simple explanations are very helpful.
same
Exactly!!
Same!!
I have a question is this use to make fake food????
I am good at chemistry
but I need help from time to time, these videos always helps me (and other member of my family)
i watched hours of videos to learn this after ncert but you cleared all my doubts in 10 min.God bless you thank you
same bhai
I resent ncert
@@huwballbot3978 kar le beta schools exams mein wahi se aaega
@@ayushroy3514😂correct
thank god this channel exists. this is going to be the sole reason I pass chem
my first time using organic chem tutor for organic chem
like frrr
I literally took this lesson yesterday at school and I did not understand it well. You came on time bro
Your FBI agent is doin a good job!
That's what she said...
@@aliidress5619 lol we learn this in the 11th standard in India
Dude you are insane, you helped my grade in Chemistry maintain in the 90s. My teachers always skip on the important parts causing us to question ourselves more than understanding the material. Keep doing what you are doing bro!
This guy had videos in the 90s??? Did he put them on vhs tapes lmao
@@matthewmealing1187 I think he means that his grades are at the 90s category
@@matthewmealing1187 he mean 90 %
@@koushik174 he was making a joke lol
I have a question is this use to make fake food?
Absolutely amazing how weeks of lectures could be succinctly stated into a 10 minutes video and can still be understood!! Thank you so much for this video!!!
I truly hope you read this because I have used your videos initially for help in my math class and then in my physics class. Now I am in organic chemistry, and I learn more from your videos, in a lot less time, than reading the entire chapters and text. A big thank you for all you do.
The most popular Online tutorial that has saved millions of student in all level Thanks bro we love you Here from Nigeria
The electronic configuration of carbon is 1s2 2s2 2p2. Coz carbon had 6 electrons.
So one electron from 2s2 will go to the vacant p orbital and form the hybrid orbital which is sp3.
So 1 2s orbital and 3 2p orbital will participate !
Except this your entire explanation was lit.
Thank you 😊
Thanks so much for this clarification; was confused about this!
I came to the comments for this. Thank you
I was wonder about this too. Thank you!
That’s what he said in the video
Thank you! what confused me was 2s drawn as having two electrons during the part where the hybridization of carbon was being discussed. I thought I had maybe missed something while watching the video.
The amount of good deeds youre getting man we absolutely love you
help i understood this way better than my prof's 2-week lecture 😭 thank you so much for saving me just before my long exams 💗
bruh 2 weeks to go over hybridization lucky i got an hour and a half lecture
@@gagemal5 what the fuck I'm learning this shit rn freshman high school
@@dastealthoperator4138 lmao I did too but beleive me it’s a lot more complicated in college than freshman high school
@@gagemal5 then I’m screwed cause idk what is happening with hybridization. I get the concept but I don’t know how to actually like calculate it lol
Hey I'm an army too
YOU DON'T UNDERSTAND. I THINK I'M IN LOVE WITH HOW MANY TIME YOU'VE SAVED ME 😶😭
😂😂🤭
R u mad?
@@Cm00987 lol, are you
😂😍
Shadi kar le😂😂
I cannot express enough how much grief this video has saved me. Thank you so hecking much, I'm certain that this is the best video on UA-cam that covers this content. Straight to the point, no fluff, just facts stated clearly. Perfect
You taught me an hour and a half class period in 10 minutes. I can't thank you enough
Gotta say it, 10 min worth of watching.
The explications are so simple yet enough
Professor Organic Chemistry Tutor, thank you for another fantastic explanation of Hybridization of Atomic Orbitals in AP/General Chemistry. The examples/practice problems continue to increase my understanding of sigma and Pi bonds.in a given molecule as well as determining if a carbon is sp, sp2 or sp3 hybridized. Thanks to the viewers for finding and correcting the error(s) in this video.
I have read chapter in my chemistry book about it, watched 3-4 videos about it and you were the only one, that made me understand it, thanks a lot!
45 seconds in and you've already cleared some things up. My textbook took like 4 pages to say that. Thank you
I have an exam tomorrow, thank God for him cause I understand more topics than I did this whole semester
His voice is very soothing and relaxing which helps more
This was a great video, I got it faster in this 10min video than my 2 hour lecture. Thanks!
The joy I get when I search a topic and see your solution is much more than d joy even my girlfriend would be capable of giving me 5yrs steady 😂❤️... I love d way u explain and your commitment to sciences... Much love from Nigeria ❤️❤️. You actually saved my first semester examination
Literally my predicament right now 😂
Ur amazing ,short and precise explanation 😁😁
Ur fan from Ethiopia
Still doing a great job after so many years! Keep it up!
8:02 man that sentence cleared up my confusion, thanks so much!
I want to hear him say "hello everyone, this is your daily dose of internet"
they are the same person????/
@@sweettoiletpaper1875 no
they aren't the same but it's gonna be lit!
This comment made me laugh. The number of times I've heard the daily dose guy talk and this chemistry tutor talk, now I'm intrigued.
Based gawr gura enjoyer
Bro is a life Saver.
Best Chemistry teacher in the world U have saved me alot❤❤
“It’s harder to break three pencils than one.” Dude I freaking love you, please keep up the great work!! You are gonna get me my bachelors!!
The Organic Chemistry Tutor literally makes everything easy pZ :)
How brilliantly he explains chemistry, mathematics, and many subjects at the same time💙. I think these videos should be translated into Arabic
When I was taught in class I didn't understand from start to end just after watching this video I'm sure if they give me a test on it right now I'll answer it well.Thank you so much.
YOU DON'T UNDERSTAND. I THINK I'M IN LOVE WITH HOW MANY TIME YOU'VE SAVED ME
Bro explains things in 10 min and in easy way.Whereas our teachers can't even explain anything easily in 40 min class period.
You are so good. Your 10 minutes video is more understandable than our 2 hrs lecture
When he did the electron configuration of Carbon, shouldn't there only be 2 electrons on the p level? Even if the carbon was to absorb another electron, promoting one of the electron from 2s into 2 p, that would only leave 1 electron on 2s and 3 on 2p
It's probably a mistake
It's obvious
Nice observation
Yes
I caught the same thing and got scared 😂
Yes ! And that’s why the 3 p orbitals and 1 s orbital overlap and form hybrids. Because none of them is full
According to Humd’s rule
Who's watching in 2024 🖐️🖐️
I Am
2024 gangggg
crying
🤚
THANK YOU!!!! now, I know why they get promotions and demotions. I am confident on this topic now. God bless
Saving my life rn 🙏
I read this topic and it wasn't too clear....so many things I didn't understand...but you made everything clear now....thank you so so much...very lucid
The king has saved me once again
Thank u so much. I was searching on the Internet abt 'why can't carbon form a quadruple bond with another carbon' and the answers I got were confusing until I saw this vdo. Thanks a million.
Didnt catch the ans for that..Why is that?
@@himanichunduru1102 Bc it only has three p orbitals, so the max number of bonds it can form is 3
@@jans3067 CH4?
thank you for single-handedly saving my chem grade
You have made possible the careers of thousands of people.
these videos are saving my college chemistry grade. thank you so much
God bless you thank you .....I was so stressed because I can not understand it very well...But in 10 minutes you explained everything. Thank you. Thank you🥀
thank you so much! You are literally saving my grades!!
Your teaching is very important so much,I love to have more updates on your channel
Man deserves a noble prize
Studying before hand and watching this has saved me
Good bless you , you don’t know how much you’re helping us ❤❤
man you have no idea how much your videos have helped me. thanks a bunch 🙏🏼🙏🏼
How is this guy able to explain this so easily??
been using you since gen chen 1. i’m in o chem now and i watched this vid in gen chem one and i need it again to review in ochem
Ready to make tiktok fan edits of this man fr ❤
I'm doing A level at the moment this stuff is so cool!!!
Thanks you really give me a simple way to understanding
you are the best chemistry teacher ever, thank you soo soo much!!
SIR I'M WATCHING THIS FROM INDIA.
Hello i m also
Thank you. I understand this so much better now. Keep up the great work and God bless.
Please organic chemistry if you see this comment please give us some examples on how to determine the hybridization of compounds and ions.
Best explanation in the whole youtube about hybridization
I'm already a sophomore in college and I still understand your videos better than my Prof's 😬
Well...I'm a junior in high school we learned this in sophomore year!
@@novazach972 good, don't ever forget it 👍.
@@novazach972 chemistry comes back in all forms of education. even grad school
@@seventhrhapsody6424 I will not my dear friend
@@zahsum I will keep it in my mind
Every day I learn more and more from you, God bless u
You're the only reason I make it through school.
tysm you're doing god's workkkkk
Man deserves a nobel peace prize for real
JUST HAD MY FIRTS ORGO CLASSSSSSS!!!!!!!!!!! I can’t believe it after all of the years of watching your videos 🫡🫡🫡🫡
Thank you sir understand 😊
nooo, you're charging now :( your videos are a lifesaver!
i’m literally so sad but tbh he deserves that coin. he’s too good at this to be doing it for free 😭
im sad to coz i cant afford it but am happy for him its about times he was doing it for free till now he deserve it
Really my sweetheart
best chemistry tutor👍👍
You are amazing, my school teacher can’t explain like this
Waoo that is awesome. I grabbed it easily. Nice job Sir
This guy dey give me joy aswear
Ur the best bro ❤
He just grippened some of my concepts.
The last few statements just cleared everything up for me wow, thanks :DD!
Nice video. Just one small critique -- please note the difference between Greek letters (symbols) sigma and theta -- your sigma looks more like a theta. Thanks.
You are genius sir... Thanks for all the videos.. it helped me a lot
You are simply the best,thankyou so much 💓💓😊😊❣️❣️
2:33 wrote 2p3 for carbon instead of 2p2 and I was so confused for the longest time 😭😭😭
Pray for me
If Organic Chemistry Professor has 1 million fans, I'm one of them. If he has 100 fans, I'm one of them. If he has 10, I'm one of them. If he has 0 fans, there's been an apocalypse.
Bro who are you dudee!! I wanna worship youuu! U just made everything easyyy dudeeeeeee❤❤❤ keep up the good work broo😊
Thank you sooo much!!!! And we need a face reveal one day 😌
bro lookup his name, he's pretty famous
@@shravjally4896 what is his name?
@@nimoabdi6230 dude, just lookup the organic chemistry tutor, and a website will open up, dumbo 😂
He's a rather obese guy with glasses
@@shravjally4896 doesn't matter, he's still hot
thank you veryyyyyyyyyyyyyyyyyyyyyyyyyyyyyyyyyyy much now iam satisfied to cover the exam
thanks! Got an basic overview in a very short time!
It helped me a lot
thank you, this video helps me to understand better!
You are one in a million sir thanks very much sir for the lectures
i love your presentations they make my life easy
love u man if u ever need anything just let me know and i got it for ya
Within the first minute everything my tutor tried to explain in 2 hours made sense
Explanation is so simple and interesting
this's just sweet Iove the voice n the way u explain everything ,, please make more videos for us ,, please 🙏🙏🙏
I really need to know if most of the people here are highschoolers or college students
because I'm learning this in high school and honestly this chapter is such a new and challenging thing for me to learn