What Is The Electrochemical Series | Reactions | Chemistry | FuseSchool
Вставка
- Опубліковано 16 вер 2024
- Learn the basics about the electrochemical series, as a part of the reactions topic.
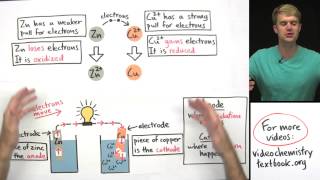
Different combinations of metals produce different voltages and this depends on how strongly the metal could force its electrons to move across a cell. The electrochemical series is a list of metals and other substances arranged in rank order of how easily their atoms may lose electrons. The further apart the metals are in the electrochemical series, the higher the voltage produced across the cell. Electrons flow along the wire from the metal higher in the electrochemical series to the metal lower down.
The electrode potentials are arranged in the substances ability to donate its electrons; that is, how easily they are oxidised. All of the values are measured in Volts.
Hydrogen has an electrode potential of 0V. The significance is that this is a reference. All of the other electrode potentials are measured against this value.
The electrons always move from the metal with the more negative electrode potential to the more positive electrode potential.
You can also use the electrochemical series to predict a displacement reaction when a metal from the electrochemical series is mixed with the ions of a metal lower down in the series. The atoms of the more reactive metal push their electrons on to ions of the less reactive metal.
The higher up the metal in the electrochemical series is the one that always displaces the ions of the lower down metal. Metals lower down in the the series cannot displace more reactive metals from their solutions. You can use this idea to predict whether a displacement reaction will occur.
SUBSCRIBE to the Fuse School UA-cam channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
JOIN our platform at www.fuseschool.org
This video is part of 'Chemistry for All' - a Chemistry Education project by our Charity Fuse Foundation - the organisation behind The Fuse School. These videos can be used in a flipped classroom model or as a revision aid. Find our other Chemistry videos here:
• CHEMISTRY
Twitter: / fuseschool
Access a deeper Learning Experience in the Fuse School platform and app: www.fuseschool.org
Follow us: / fuseschool
Friend us: / fuseschool
This Open Educational Resource is free of charge, under a Creative Commons License: Attribution-NonCommercial CC BY-NC ( View License Deed: creativecommons... ). You are allowed to download the video for nonprofit, educational use. If you would like to modify the video, please contact us: info@fuseschool.org








why are the electrons sad tho? :(
Best! Wish other videos explained the chart it would have helped plenty with understanding for instance how a battery works
best explanation ever
Thank you!
Thank God there is a nice explanatory video of electrochemical seriesin whole u tube🤓🤓
Thank You so much, It helped me a lot
My all Concepts are clear now
Thank you so much ❤️❤️
You're most welcome 😊 always happy to help!
Thanks The Fuse School. Quite helpful.
Can you also explain what happens at the salt bridge in molecular scale please?
Thank you! Very clear and concise
thnqq so much ..this video made my concepts very clear
It reallyyyyyyyyyyyy helps me a lot thank you
You're most welcome!
really helpful...awwwsssooommmmmeeee
Hi can you suggest the best way to memorise it pleaseeee.,thank youuuuu
if Li is more electropositive than K,then it's supposed to be more reactive than K but we see that lithium in water yields less hydrogen and heat than K,
Why is that?
because Li is less reactive than K. this is because valence electrons in K are more far away from the nucleus of the atom, so they are less attracted to the nucleus, therefore being more prone to let go of the atom. therefore, K is more reactive than Na, and Na is more reactive than Li, also Ca is more reactive than Mg and so on. hope this helped
thanks
2:12 ... logic: look at the beakers from the other side.
really helpful video
thanks!
good
this really helped me!
Glad it helped!
It is really helpful
Glad you find it helpful!
Thank you so much
No problem!
Nice explanation of the problem 👍👍👌👌😁
Glad you liked it!
Tq so muchhh ......wish me a good luck in my final😖
Hope it went well!
Thanks!
No problem!
Where is lithium can you explain plz
❤️
@@fuseschool hi fuse school!
Hi Khosel!
@@fuseschool can u clear a doubt of mine? Electrochemical series are arranged in decreasing order of oxidising nature or in decreasing order of reducing nature? Nobody is sure about this .. can u clear this doubt of mine? Thank u
@Khosel Normally the table is arranged with actual metals (or elements), not their ions, with the most reactive (e.g. Cs or K) at the top, so decreasing order of reducing power.
best explanation ever