18.1 The Laws of Thermodynamics | General Chemistry
Вставка
- Опубліковано 7 лют 2025
- Chad provides an introduction to Thermodynamics describing the Three Laws of Thermodynamics.
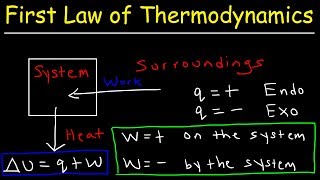
The First Law of Thermodynamics, covered originally in chapter 5, can be summarized as stating that energy cannot be created or destroyed.
The Second Law of Thermodynamics states that for a spontaneous process the entropy of the universe increases.
The Third Law of Thermodynamics states that a perfect crystal at zero Kelvin has zero entropy.
I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at www.chadsprep....
If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course at www.chadsprep....
00:00 Lesson Introduction
00:23 1st Law of Thermodynamics
03:23 2nd Law of Thermodynamics
07:37 3rd Law of Thermodynamics
www.chadsprep....



![Lp. Сердце Вселенной #60 РОЖДЕНИЕ ЛОЛОЛОШКИ [Финал] • Майнкрафт](http://i.ytimg.com/vi/YoR0pAV9FVQ/mqdefault.jpg)




CHAD DOING THE LORDS WORKKKKK! Thank you for being such an excellent educator.
You're welcome and Thanks for all the comments!
You are doing something very valuable and everyone should be grateful to you for that
Thanks for saying so!
blessed efforts professor :)
Many thanks
You do a great job at getting these points across. Thank you!
You're welcome - and thank you!
Thank you Chad. I have learnt a lot from your videos. You are an inspiration. I am a high school Chemistry teacher and you have inspired me to seek for ways to take stress of learning Chemistry from my students
You're welcome - Glad to hear the videos/channel are helping you - much success to you and your students as you teach.
Thank you Sir.
Another banger from my main man, Chad! We're on track to finish 1-a and 1-b strong.
Excellent!
Bro u dont know how ur helping me right now hope ur doing amazing❤
Glad the channel is helping you!
Hi Chad, have you heard of Ginsberg's version of the laws of thermodynamics:
0. There is a game
1. You can't win.
2. You can't break even.
3. You can't even get out of the game.
I like it Peyman! :)
When the temperature goes up, randomness goes up. That's why people get all crazy when the weather is like 100+ degrees.
Thank you Chad! You did an amazing job, subscribed!
You're welcome and Thank You!
I owe you my chem 2 grade - thanks for all of the amazing content
You're welcome.
Thank you sir... 🤝💥
Great work
Thanks James!
I think I am in love with you in an academical way, sir.
Glad you find the channel of value - Happy Studying!
really helpful thank you very much
You're welcome!
Thank you sir
Welcome
great video
Thank you.
Omg thank you!
You're welcome!
Nice sir. Life is good!
Great!
you the best sir,continue helping us.Very much appreciated work
You're welcome and Thank You.
Interesting 👍
Happy Studying!
Any good UA-camrs to watch for Bio 2
I’ll second that request! Gen Bio 2 help!
❤
Thanks!
arigato osaimastha
You are most welcome.
Hii
Welcome to the channel.
No offence, Chad, but this reminds me of The Emperor's New Clothes-link here( ua-cam.com/video/ZC38jekCaB8/v-deo.html ) for those who haven't seen it.
So where do the surroundings and the system come from? The law of thermodynamics tells us straight off the bat that neither the surroundings nor the system could begin from nothing, but that is what evolutionists would have us believe.
Why do he write 0 like this? ∅?
Sorry, it's a habit I developed long ago so that students could more easily tell the difference between the letter 'O' and a zero. Hope it helps more than it is distracting 😊
@@ChadsPrep no it's not distracting, I was just curious so asked! And you reply so fast 👍😲
@@Aakash-e1x4t Not always but sometimes it just works out.