Le Chatelier's Principle
Вставка
- Опубліковано 14 лип 2024
- 066 - Le Chatelier's Principle
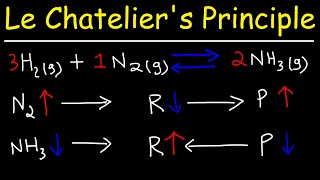
In this video Paul Andersen explains how Le Chatelier's Principle can be used to predict the effect of disturbances to equilibrium. When a reversible reaction is at equilibrium disturbances (in concentration, temperature, pressure, etc.) will be offset to reach a new equilibrium. For examples when more reactants are added the reaction will move to the right to reestablish the equilibrium constant.
Do you speak another language? Help me translate my videos:
www.bozemanscience.com/transla...
Music Attribution
Title: String Theory
Artist: Herman Jolly
sunsetvalley.bandcamp.com/trac...
All of the images are licensed under creative commons and public domain licensing:
Eframgoldberg. English: An Overlay of the Same 99.9% Pure NO2/N2O4 Sealed in an Ampoule. From Left to Right -196C, 0C, 23C, 35C, 50C, July 16, 2013. Own work. commons.wikimedia.org/wiki/Fil....
en:User:Greenhorn1. English: Nitrogen Dioxide (NO2) on the Left and Dinitrogen Tetroxide (N2O4) on the Right., February 25, 2008. en:Image:N02-N2O4.jpg. commons.wikimedia.org/wiki/Fil....
"File:Ammonia-3D-vdW.png." Wikipedia, the Free Encyclopedia. Accessed January 3, 2014. en.wikipedia.org/wiki/File:Amm....
"File:Dinitrogen-Tetroxide-3D-vdW.png." Wikipedia, the Free Encyclopedia. Accessed January 3, 2014. en.wikipedia.org/wiki/File:Din....
"File:Tetrachlorocobaltate Aqueous Ion.jpg." Wikipedia, the Free Encyclopedia. Accessed January 3, 2014. en.wikipedia.org/wiki/File:Tet....
yinch. English: SVG Version of Nitrogen Molecule., November 25, 2010. Produced in Inkscape. commons.wikimedia.org/wiki/Fil....








mr Anderson we are indebted to you
I love your style of teaching. Soo much better than my chem teacher, who introduces something to us, barely explains, and expects us to be able to take a quiz on it the next day. >.>
Sounds like a lot of teachers... Very few like this guy!
Sandra Gonzalez lol, my teacher doesn't know how to teach either, to top it off we have a quiz on the same day he teaches us a new topic...
my teacher acts like were stupid when we ask questions!! im not a chemist how would i know the answer. love this guy
mine does the same but then refuses to answer questions on it or go through it again come exams.
Our*, not "Are". What english class do you have by the way?
You just explained something to me in 7 minutes that I would have not understood had my chemistry teacher taught it to me. It was very helpful, thank you!
Why are the best teachers always on UA-cam?
my ap bio teacher made us watch your videos as assignments and i'm still using your videos for years later in my college courses... you have helped me in my journey as a science major tremendously!! thank you.
same!!
thanks vro its 9pm and im doing chem hwk
3:17 am march 18, 2021 mid pandemic. Hello to the future students reading this comment. Its hard but youll learn it like he did and I did. Anyways tryna imagine life post pandemic wonder what that will be like. Anyways future student gotta go
Back go studying
Wherever you work, Sir, whatever you get paid, you should get a raise. Thank you so much for all the efforts you put into your yt channel :-)
Your chem videos are almost exactly in unison with my ap chem class. Thank you
jus saying eli manning best qb ever now stfu
it is Tom Brady
You literally make everything so clear! Im currently studying for the MCAT and your videos have made the process so much smoother! Thank you Mr Anderson!
You explain the concepts very clearly and your animations really help in visualizing the concepts. Thank you for being such a great educator!
Even in college, your videos are still as helpful as ever! Thank you so much!
OMG I love this man.. I needed to know this for my chemistry lab tomorrow :D
You're the best! Thank you for making this video and the other chemistry and biology videos. They're all very helpful!
Thank You, the visual diagrams help a lot. It actually makes sense.
When you showed the actual implications with percent yields that made it all so much more clear.
Thank you man. I hadn't understood this in more than two years. Thank you *cries*
Thanks so much! I have a chem test on this stuff tomorrow and I'm racking my brain over these shifting concepts. That definitely cleared things up!!!!!
Thank you, Mr. Anderson. Always helpful, since AP Bio to General Chemistry 1 & 2.
We’re deeply grateful for your help
This helped me so much! you explain everything so well! thank you!
This goes hand in hand with the fact that when enthalpy is endothermic (delta H is greater than 0), then when it has a lot of temp it’s spontaneous (goes to the right) and when it lowers temp, it goes to the left is non spontaneous.
oh my God this is awesome everything start to click. i have been stuck on this for a week and thats studying it everyday
this is my new favourite channel.
I enjoyed you video. You explained the topic simply and thoroughly. Thank You.
Thank you so much. For everything I am one of your fans. I wish that you enjoy your year off and keep doing this excellent thing. I could try to translate your videos to Spanish,
let me know if you are okay with that!
This was very helpful you did such a great job at explaining it thank you so much!
you are among the best teachers for ever
I like your channel and you have been a tremendous help in my success. Please continue on. Thank you!
Thank you so much Mr. Anderson...this helped me greatly :D
Btw, I also like your process of teaching: very straight forward, yet simple enough to apply and follow along :).
Saving my life all the way from High School Ap Bio up until now in Chem 2 in college...thx chief.
Thank you so much all your lectures are really helpful.
THANKS SO MUCH! I was so confused. i take AP Chemistry and i was so confused about Le Chatelier. Thanks for spelling it out for me.
thank you , i learned a lot :D I couldn't understand to my teacher, so I found you, and it was AWESOME! ;D
I should have watched this 5 years ago, thank you for the help.
This video from when I was still in 5th grade is now very usefull. Thank you very much 💗
You are so good. Such great explanations. Love it
Thank you SO much! This video was super helpful!
Thank you so much for a wonderful lecture.
Wah! Wonderfully explained! Thank you very very much!
Perfect explanation :) Thanks a million
simple and straight foward. i only know of the temp and pressure part. knowing the concentration helps also.
this was life changing. thanks a lot
Thank you, this was so helpful!
I love your videos so much. Thank you!
In any reaction where it is reversible and we have reactant and products and equilibrium is reached, if there is ever a stress factor involved like increase/ decrease temperature, increase/ decrease pressure there is going to be a new equilibrium will be established. Increasing yield by concentration when we want more product we raise the concentration of the reactants to shift reaction towards the right and vice versa.
Thank you, this was very helpful.
Any videos explaining how to determine Q#'s of different elements? Thank you Mr. Anderson for being super duper awesome! :)
I think I now know how to make my slides. Thanks Mr Anderson
Wow. This video is amazing.
Great video. Thank you.
this was so helpful. thanks.
Loved the video..Thanks Sir!!
Very easy to follow, thank you!!!
Keep going
I wished all our doctor like you 😪
thank u so much sir love ur way of teaching
Thank you so much!
Great explanation
Wow sir !!!! impressive teaching style ☺tq sir
You are great! Thanks
It was helpful, thank you...
great video
bozeman you should teach math from the ground up all the way to pre-calculus, i would watch that more than chemistry and biology, dont know about everybody else
Hey Great video however, with the reaction of N2O4 to 2NO2 when you increase pressure or decrease volume why would the reaction shift right first and then go to the side of fewer moles? I thought that it would shift left to reactants first and stay there till the volume would allow the product of nitrogen dioxide to form? A little confused
Thanks :D Really love the video you made :) I survived CHEMISTRY ;D
amazing!! thanks a lot!!!
So helpful...... Thank you
Thank you!
thanks ....quite helpful
Wish you made all these videos last year :(
so helpful!
thank you sir
Thank you very much!!!!!!!
Holy Moly, thanks you so very much! I'm taking General Chemistry 2 in the summer and my professor just threw this on to us and expected us to know it by the next day... I salute you for making my science journey possible. THANK YOU! Also do yu have a video on how to memorize the acids/bases ?! I confuse them all..
Navneet Kaur there are others but I don't think he has one
lol, thank you for your reply. i actually understand it now and i passed my chem 2. now taking organic chemistry. FUN STUFF!
wow! How is it going one year later?? Hopefully well :)
Really noice video thank you.
your the reason I understand chem... thank you so much
But you don't understand english!!!! You're* ezpz
THANK YOU
Thank you
thank you!
good video!
Sir, You are the best. Regards from India. My Chemistry teacher sucks !!!! :D
Anyone here cause all there classes are online now?
OG_DanTheMan literally me taking notes on this for my Chem class rn
Amazing.......sir
about to take my ap chem exam! thank you!!
that's helpful❤
Thnk you for helping me pass gen chem
Am I the only one here who has a great AP Chem teacher?
I had a good one
Is the value of K in the new equilibrium going to be the same for the same temperature, or will there be a new K value?
To all the chemistry god's you are one of the best.
Far superior than my professor's explanation.
how can I ask you questions mr anderson about other topics that you haven't yet uploaded videos about 'em
Your stuff is good for the general concept but i think it would be more effective if you showed difficult examples. Good video though
You are an angel
When more of a reactant or is added, will the new equilibrium have a different Kc (equilibrium constant) than our original reaction? Or will it remain constant?
Kc depends on Temperature
Great....
Thanku sir!
My teacher should not be teaching chemistry so thank you for this video !!!!
Thanks for saving me. My chemistry teacher wont even know this..
Could you add examples of application that would have been helpful. Thanks
After we place a stress on the reaction (by removing/adding reactants) and allowing that reaction to come to a new equilibrium, does the new equilibrium still have the same equilibrium constant? Afterall, it is referring to the same chemical equation.
Yes, since for a given reaction, at a temperature, Kc stays the same. The only thing that changes is Q. When the equilibrium is thrown off, the value of Q will change (in other words, Q no longer equals to Kc). According to Le Chatelier's principle, the reaction will shift in the direction that restore the equation: Q = Kc.
bruh u trippin??? u a genius