How To Balance Redox Equations In Basic Solution
Вставка
- Опубліковано 18 чер 2016
- This chemistry video tutorial shows you how to balance redox reactions in basic solution. The first step is to separate the net reaction into two separate half reactions - Oxidation and Reduction. Balance the atoms first under acidic conditions using H+ and H2O and then balance the charges by adding electrons to the side of the chemical equation with the highest oxidation state. Once the electrons in both half-reactions are equal, the two reactions may be combined together to form the net reaction. Add OH- ions to both sides of the equation to neutralize the acid - this is how you can balance the redox reaction under basic conditions.
Stoichiometry Practice Test:
• How To Solve Stoichiom...
Solute, Solvent, & Solution:
• Solute, Solvent, & Sol...
Strong & Weak Electrolytes:
• Identifying Strong Ele...
Molarity Practice Problems:
• Molarity Practice Prob...
Ion Concentration In Solutions:
• Ion Concentration in S...
Dilution Problems:
• Dilution Problems, Che...
___________________________________
Types of Chemical Reactions:
• Types of Chemical Reac...
Solubility Rules:
• Solubility Rules
Predicting The Products of Reactions:
• Predicting The Product...
Activity Series of Metals:
• Activity Series of Met...
Will This Reaction Occur?
• Chemistry - Will The R...
Predicting Products of SR Reactions:
• Predicting Products of...
___________________________________
Double Replacement Reactions:
• Introduction to Double...
Net Ionic Equations:
• Precipitation Reaction...
Writing Chemical Equations From Words:
• How To Write Chemical ...
Solution Stoichiometry:
• Solution Stoichiometry...
Molarity & Dilution Problems:
• Molarity Dilution Prob...
Acid Base Neutralization Reactions:
• Acid Base Neutralizati...
____________________________________
Acid Base Titration Problems:
• Acid Base Titration Pr...
Mixture Problems:
• Mixture Problems
Calculating Oxidation Numbers:
• How To Calculate Oxida...
Oxidation and Reduction Reactions:
• Oxidation and Reductio...
Balancing Redox Reactions:
• Half Reaction Method, ...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
___________________________________
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections


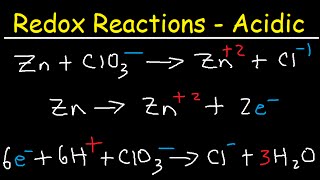

![Lp. Последняя Реальность #107 РОДНОЙ ДОМ [Финал] • Майнкрафт](http://i.ytimg.com/vi/IK3QKzKUlHM/mqdefault.jpg)




Final Exams and Video Playlists:
I genuinely hate school
Finally he has reached 1M subs! deserved it!
I'm terribly happy because I finally understand how to do this
I always had problems when balancing with OH-. Never thought about balancing with H+ first. It worked!!!! Thank you!
Balancing using acidic conditions, then adding OH- ions to convert it to alkaline conditions is so much more smart and efficient bruh
ARE YOU STILL ALIVE NOW?
Best tuitor🫡
7.23 million now
well then. You can just balance in acidic conditions and then add OH- ions for basic conditions huh? Guess what. Works EVERY time. Mind officially F#$&ING blown. Bugger ever starting in basic conditions EVER again. Thanks buddy.
THANK YOU!!! I've been struggling with balancing redox equations in basic conditions and this actually helped me so much!!
Thank you so much! I've been struggling with this for the last 3 years and now you have solved this!
These videos are amazing!!!! You always display great tips , this was extremely helpful thank youuu 🙏🏽🙏🏽
you're the reason why i understand this redox. i really enjoyed the progress of learning this despite from being time consuming for every problem, atleast i became happy whenever i solved it. i have nothing to say but thank you.
This video was really helpful! Everything became clear after two weeks of neverending suffering from the confusing discussion of the ion-electron method! Thank you, sir!
U are the best, anytime I find problems hard u always have easier solutions to them
love the videos, this is my last unit before finals....cant wait to be done with this stuff
Thanks so much, you make chemistry look so much easier. More grace to your elbows.
Most reliable channel! You make chem look so easy. I'm saved.
ha you're too good man, love your videos! Got me through chem 1 and chem 2!