Stoichiometry with Mass: Stoichiometry Tutorial Part 2
Вставка
- Опубліковано 14 гру 2016
- This is a whiteboard animation tutorial of how to solve Stoichiometry problems involving mass.
For a limited time, get $200 cash if you qualify! Check out:
www.referyourchasecard.com/18...
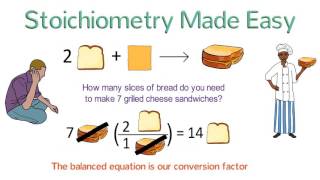
Because amounts MUST be converted to moles before converting between chemicals, stoichiometry problems involving mass are muti-step calculations. This video explains how stoichiometry with mass works for an everyday problem involving food and then goes on to show how to solve a 3-step mass-mass stoichiometry problem for a chemical reaction. This is my second tutorial in the series.
Steps for a Mass-Mass Stoichiometry Problem:
1) Write down the balanced chemical equation
2) Calculate the molar masses of the chemicals
3) Convert mass to moles of what you know
4) Convert moles of what you know to moles of what you want to know
5) Convert moles to mass of what you want to know
My goal is to make chemistry easier ;)
ketzbook.com
#chemistry #madeeasy #stoichiometry #ketzbook #tutorial - Фільми й анімація








It’s amazing how a roughly 6 minute video can explain this stuff better than weeks of school work on it
This is really helping with my understanding !
Explained better than my lecturer.
Ty.
glad I could help!
I really appreciate that you explain why each step is done the way it is. My biggest problem in math is not being able to set up equations because I never understood why certain equations are set up the way that they are. This video has helped me to understand, and I could not be more happy about it! Thank you!!!!!!
you're welcome!
1.) balance reaction 2.) get molar masses 3.) convert mass, grams to moles 4.) convert moles to moles 5.) convert moles to grams, 00611
i have found out that i am incapable of learning this
You surely are CAPABLE of learning this or wathever thing, just believe in yourself.
@@cientifica9150 me and you bruv
@@ashleymowver8633 What does it means "bruv"?
@@cientifica9150 british bruh
@@mimaii oh okay, thanks
Thank you sooooo much, this helped me a lot.
Thank you so much! This has made stoichiometry a lot easier to understand. You've gained a sub :)
You absolutely right, same here
shut up
Omg thanks, I still don't totally understand, but it's better now woth you video
Thankyou so much sir.... You teach in a very simplified way that anyone can get☺
im a chemist and i loved this hahaha, ill try to teach like this. ill include foods also, thanks
Great!
Thank you so much, I really appreciate this video! I've watched many youtube videos about Stoichiometry but I've never really gotten the hang of it! but now that you've explained it step by step made me fully understand the concept! Thank you, you've gained a subscriber!
glad it helped!
You made this so easy, thank you.
watchin just before my exam , million times better than my teacher . understood well
So happy to find this channel😄
Great video! The food analogy makes it so easy to understand! I know I will keep my 4.0 for sure!
Did you keep it?
thanks, helped me out a lot
Bro this video was so much more helpful then my 2 hours of class that I’ll never get back
THANKS I can finally understand the concept of this
A good trick to remember when solving is the number outside the conversion factor is divided by the bottom number inside the conversion factor 122095
Thank you for your videos you’ve gained a new sub 😊🥰
Hello girl
Wow that's great
We can easily find by taking the mole fraction of the above question
Thank you, you are better than my teacher lol
Very helpful !
THANK YOU SO MUCH
thank you so much it really helped!! :)
You're welcome!
I can't thank you enough.
life saver! thankyouuu 🙂
you're welcome!
you my man are super amazing
Thank you for your information:)
Hello beauty
lifesaver tbh ily
00815: 5 Steps for stoichiometry are write a balanced equation, determine your known and unknowns, covert mass to moles, convert moles to mole (ratio), and convert mole to grams
1.) Converting from one chemical to another you must use the units miles and grams
2.) I learened that to convert moles to miles you divide by the amount of elements itself.
00801
Good stuff. It made me hungry too. :)
😃
1- write down chemical reaction, 2- calculate the molar mass of chemicals, 3-convert moles to mass of what you know, 4- convert moles of what you know to miles you want to know, 5- convert miles to mass you want to know. 00403
the conversion factor between moles and grams is a molar mass is the atom or molecule. 00104
Question: What are the 5 steps for stoichiometry with mas?
Answer:
1. Balance the reaction
2. Find the molar mass
3. Grams to moles
4. Moles to moles
5.Moles to grams
Question: What is always in the middle fraction when using a 3 conversion factor fraction?
Answer: Moles
ID: 00807
Thank you!
welcome!
Thank you
Wow you are reall teacher
yes, and I'm still teaching, thanks!
the conversion factor between one chemical and another is the weight of the reactant that you is given and then put the reactant that you are loving for on top it can also be the balanced equation, 00214
Man , you're making it a lot harder
harder than....what? The math involved for any method will always be the same. The main difference between methods is the organization of the numbers and units. What I presented in this video is the standard method, and frankly chemists of all levels use this because it minimizes mistakes (people make mistakes frequently when they try to just multiply this and divide that in their calculator without writing it down). In the third video in my series, I highlight several tricks and patterns that make it easier to remember this set up.
The balanced reaction is used as the conversion factor. 00105
1) balance reaction
2) get molar masses
3) convert mass, grams to moles
4) convert moles to moles
5) convert moles to grams00810
Hi Ketzbook
I would request you to please provide us some questions on this topic and please reply ASAP.
I loved it how you taught and understood the topic
Thank You
Love and Good Luck From India
update: bro did not reply asap
1) balanced reaction2) molar mass3) grams to moles4) moles to moles5) moles to grams
please include your id#
1.) because it has to go from grams to moles
2.) the balanced equation is the conversion factor when doing moles to moles, the coefficients in the equation tell the ratio of the chemicals within the reaction
please include your id#
00414
The conversion factor is the ratio or fraction( using moles)
hello to you thanks of you its so so so goods
00803)
1- Record and balance the chemical equation
2- Find the molar mass for each element
3- Convert grams to moles
4- Convert the moles of what you know to what you want to know
5- Convert moles to grams
This is really helpful 👍 Thanks a lot!!
But to make the calculation easy,by reducing steps, there's another method to calculate which gives the same answer
i.e, 100g of H2O×16g of O2(considering only one molecule)/18g of H2O =88.8 g of O2.
Hence,the answer is same can this method be applied too?
yes, see my next video about combining the steps
ua-cam.com/video/-1xfnq8yGk8/v-deo.html&
@@ketzbook Thank you☺️
00123
1. Balanced reaction
2. Molar masses
3. Grams to miles
4. Moles to miles
5. Moles to grams
Too left and bottom right- 1 mol
Can you make a video about acids and bases? solutions?
00802 It makes it more difficult because there is more steps needed to solve mass. The steps go 1) convert mass to moles
2) convert moles of one chem to moles of another
3) convert moles
There are more steps and conversions when mass is involved in stoichiometry, therefore you must convert mass to moles, then the moles of one chemical to the moles of another, then finally convert moles to mass. 00421(Elijah Tull)
1.) Write down the chemical equation and balance it
2.) Calculate the molar masses of each element
3.) Convert grams to moles
4.) Convert the moles of what you know to what you want to know (moles to moles)
5.) Convert the moles back into grams
00823
ratio of fraction converting mass to moles is the conversion factor, 00812
It is more difficult because you have to 1)covert mass to moles, 2) convert moles of one chemical to the moles of another, 3) convert moles to mass. 00810
The conversion factor between moles and grams is the molar mass of an atom or molecule. 00610
ID: 00404 The conversion factor between moles and grams is (known amount of grams)(1 mol/ molar mass of one molecule)
Because molar mass is the mass of a given product (molecule) and mass is grams is a universal measurement of mass regardless of the entity measured. Molar mass is integrated for molecules while grams is derived for comparison. 00205
You can not convert from grams of one thing to another directly because it is a ratio of the mole. Once you convert into moles, you can multiply or divide and finally convert to grams again with your conversion factor, 00213
1. Balance equation 2. Molar mass3. Grams to moles4. Moles to moles 5. Moles to grams00119
Pleaxeeeee do make lectures on physical chemistry plxxxxxxx
1. Balanced reaction
2. Molar masses
3. grams to moles
4. moles to moles
5. moles to grams
00115
1) balanced reaction
2) molar masses
3) grams to moles
4) moles to moles
5) moles to grams,
00811
1. Write down the balanced reaction 2. Calculate the Molar masses 3. Convert the mass of grams to moles 4. Convert from Moles to moles 5. Convert moles to grams, 00715
1. Write down the balanced equation 2. Calculate molar masses of each chemical 3. Convert mass to moles 4. Convert what you want to know to what you want to know in moles 5. Convert moles to mass of what you want to know, 00415
1) Balance the equation
2) Find the molar mass
3) Convert grams to moles
4) Convert the moles
5) Convert moles to grams
,00419
1) Write down the balanced chemical equation 2) Calculate the molar masses of the chemicals 3) Convert mass 4) Convert moles5) Convert moles to mass. 00111
1)Write down the balanced equation
2) Calculate the molar masses of the chemical
3) Convert mass to moles of what you know
4) Convert moles of what you know to moles of what you want to know
5) Convert moles to mass of what you want to know
00607
Can't convert directly from grams because that is a ratio of moles. We use the balanced reaction to know the amount of a product that's used | 00805 |
1) Balanced reaction 2) Molar mass 3) Grams to moles 4) Moles to moles 5) Moles to grams, 00103
you must covert to moles before going to grams, what ever unit your solving for you put it on the bottom of the equation. 00721
The conversion factor is the ratio or fraction converting mass to moles , 00606
you have to convert from mass to mol or vs versa
1. Write down the balanced chemical equation
2. Calculate the molar masses of the chemicals
3. Convert mass to moles of what you know
4. Convert moles of what you know to moles of what you want to know
5. Convert moles to mass of what you want to know
00223
1)Balance the reaction
2)molar masses
3)grams to moles
4)moles to moles
5)moles to grams,984311
1) Balanced reaction 2) Molar mass 3) Grams to moles 4) Moles to moles 5) Moles to grams, 00107
1) balance reaction
2) get molar masses
3) convert mass, grams to moles
4) convert moles to moles
5) convert moles to grams
00703
I had a stroke when he said " one piece of hot dog meat"
hope you recover :)
Thanks a lot, but, the example of hotdogs is somehow difficult to understand for students. Secondly, when you make the mass to mole conversion, no need for all this complication, n=m/M, just get the number of the moles, do the Stoichiometry ratio to get the number of moles of the second molecule, and repeat n=m/M to find the mass and that’s all.
The conversion factor is the molar mass of an atom of a molecule 00112
1) Write down the balanced chemical equation
2) Calculate the molar masses of the chemicals
3) Convert mass to moles of what you know
4) Convert moles of what you know to moles of what you want to know
5) Convert moles to mass of what you want to know
Grams to moles
00407
Hey you said that the coefficient in the balanced reaction do not affect the molar masses because molar masses is the mass of only one mole of that substance.
Then the coefficient will also don't get multiply to Oxygen and hydrogen..
Please give me solution...
you have steps to go through, 1).convert mass of moles, 2)convert the moles to chemical, 3)convert moles to mass
Mass makes stoichiometry more difficult because it requires multiple steps and conversions. For these problems, you must convert mass to moles, convert moles of one chemical to moles of another, and convert moles to mass.
please include your id#
I feel like you should be my teacher please, like for real
i need a summary of what u did
The conversion factor between one chemical to another is moles. 00814
Make video on soda glass
Where did you get 1.oo8 from?
How do we solve involving mol/dm
The conversion factor between one chemical to another is moles, 00114
Calculate the molar masses of the chemicals, and then Convert mass, and next Convert moles and finally, Convert moles to mass...00412
Can you help me by showing me to solve the following problem . Calculate the mass in grams of H2O produced from the reaction of 10.0 grams of H2 with 99.8 grams of O2.
It is difficult because you need to convert the mass to moles, then convert the moles of one chemical to another, then convert the moles back to mass. 00818
Can someone explain why he put 88x(1/1)= 88, I thought that you put 0.10 since that’s the weight of the bun ? What am I missing 😅
-Mass makes stoichiometry harder because first you have to convert from mass to moles, then convert moles of one chemical to molds of another, and may have to convert moles back to mass, 00710
Mass makes it harder becuase you have to use conversion factors to get the answer , you can not get the answer until your in moles , 00110
You first have to convert into moles on order to answer the question, 00702