Titrations of polyprotic acids | Acids and bases | AP Chemistry | Khan Academy
Вставка
- Опубліковано 28 лис 2021
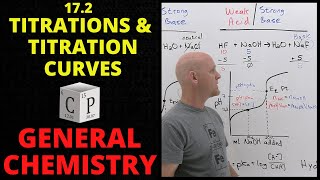
- Titrating a polyprotic acid with a strong base produces a pH curve with as many equivalence points as there are acidic protons on the acid. The pKₐ values for these protons can be estimated from the corresponding half-equivalence points on the curve, where pH = pKₐ. View more lessons or practice this subject at www.khanacademy.org/science/a...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacademy.org/donate?ut...
Volunteer here: www.khanacademy.org/contribut...








Thank you for the simple and concise explanation of these titration curves. You break it down perfectly!
First and love all your videos they all help with everything I haven’t had a problem that your videos don’t help me
I ❤️ Khan academy
Thanks so much, keep up the good work
I like the systematic approach, but I’m worried this is giving an overly simplistic view of polyprotic acids, there must be a consideration of strong/weak acid and this idea while isn’t required for a titration shouldn’t be neglected :/
thank you very much
Very clear explanation, thanks 🤍🤍
Hello. I am here from russian version of this channel. I want ask you, what program do you use for your videos? You drow with a computer mouse or with a Graphics tablet?
I hope you understand my bad-english comment and you will answer me)
На самом деле в вашем комментарии всего одна ошибка: рисовать пишется ''draw''. Надеюсь вам ответят.
Màster in Engineering here🇺🇸🇺🇸
but why the ph of HA is 7 since it is an acid?
Sir I have a doubt in Math can u help me please
Cryogeniced solutions of self sustaining value
Acid
One molll timeee
First
RIP Kahn Academy Dead
This is the dislike button.