Understanding the Power of the FDA Pre-Sub: Tips for a Successful Meeting
Вставка
- Опубліковано 4 жов 2020
- The submission process to the FDA can be a stressful and uncertain time for a medical device company. Delays in getting your device to market can cost millions of dollars in revenue. Changes to standards, new guidance documents, and review variability all add to the stress of a submission. The FDA pre-submission process can help reduce the stress and answer questions related to your specific device.
In this webinar we will cover what the pre-submission process is, how to initiate a meeting, and how to prepare and run the meeting to gain the best results. We will highlight tips we have used to make partnering with the FDA a rewarding experience.
Join Thor Rollins and Matthew Jorgensen; PhD, DABT, as they cover:
• What is a pre-submission meeting (Q-sub) with the FDA?
• How do you set a Q-Sub?
• How do you deliver your plan during a Q-Sub?
• What is the meeting flow?
• Can I challenge the FDA?
• How do I get the most from the Q-Sub? - Наука та технологія



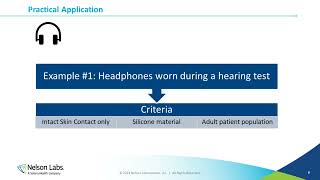





What publication style (eg, AMA, Chicago, etc.) should be used for the Pre-submission Meeting Request? I'm editing a doc for a device developer but I can't find any guidance on how the citations should be handled.