Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy
Вставка
- Опубліковано 7 жов 2024
- Keep going! Check out the next lesson and practice what you’re learning:
www.khanacadem...
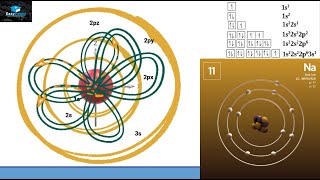
The electrons in an atom are arranged in shells that surround the nucleus, with each successive shell being farther from the nucleus. Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have different energies. View more lessons or practice this subject at www.khanacadem...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacadem...
Volunteer here: www.khanacadem...








You have taught us more than the book. Blessed to have you in life! Happy Teachers' Day
Tushar Kalamkar Which book is that ...?
@@Pi2.718I am talking about in general not about this specific lecture for eg. (Maths, physics like sub)
@@tusharkalamkar9163 I got it now :) Thank you for replying !!!
Tushar Kalamkar I swear that came just cause of teachers day
I dont mean to be so offtopic but does anybody know a tool to log back into an Instagram account..?
I was dumb forgot the account password. I appreciate any tips you can give me.
You are 1 million times more helpful than my teacher ty
😂😂😂true me too his helped me very much
Ertugrul learning orbitals is great
Believe in Lord Jesus Christ/ Lord Yahshua and Repent from y’all sins/ evil deeds
Please never stop making these videos... U don't know how helpful they are
He knows probably
I’m still so confused I hate chemistry with a passion 😍😍
I feel the same way about the orbitals, shells and subshells; especially the fact that as you "fill" the shells, sometimes you have to arbitrarily jump Down an energy level, before you go back up again. One teacher told us that you really have to understand quantum mechanics to understand the explanation as to why. I don't intend to learn quantum mechanics just to understand the logic of the order of how electron shells are filled, which means it's back to learning by rote; which is how they taught chemistry in high school: "just memorize it for the test". They literally told us that. Brilliant, teacher. That told me they didn't understand the physics behind it any more than I did......
However, don't lose heart. One day you'll find a teacher who can actually explain it so you understand it. At least that's what I keep telling myself.
The same problem is with me😅
😅
@@maskedmarvyl4774well explained
I've studies orbital 10 times but this is the first time i have "actually" understood, marvellous job Sal!
No prof has explained this as well as you have, just asked me to blindly memorize it not knowing what it means.
Thank you for clearing up years of confusion.
Isn't it extremely amazing,we all divided by the borders and united by the knowledge,came here at this particular platform to gain knowledge collecting for different exams.
I wish all the luck to the future ones who will still be coming here after months and years.
No freaking way you explained this in 10 minutes and I spent 2 lessons trying to get my head round this and try to visualise it
Exactly my situation! Yeah lightbulb within 10 min 🙂
I just came here to thank you for everything you did , you helped me understanding a lot of things like math
I got the full point in the national exam thanks ❤
KAHN Academy is an absolute sanity, life, and grade saver! I am SO thankful for all the amazing material you post! THANK YOU!!
Was fascinated with chemistry in my undergrad. Mastered just about everything you'd find up through O-Chem. 12 years later I'm studying for my MCAT.... I forgot nearly ALL of it. This is a lifesaver.
ayoo your profile pic what do you make using chemistry
@whoamiwhoareyou-of4jm I make the things you cannot. Next time, apply yourself.
How did the MCAT go?
thank you, I've been trying to understand this for a while, no other video could explain it as clearly as you, thank you. It finally makes sense.
Love u man! Best Teacher EVER! Keep the good work going! SAL IS DA BEST TEACHER, THANKS TO HIM IM A SEVENTH GRADER LEARNING 9TH GRADE THINGS!
This is the first time I've seen a video that actually explains shells, subshells and orbitals in a way that I can understand. Before it was just a confusing mishmash that seemed completely arbitrary, in the way it was taught. It all has to do with the amount of energy imparted to the electrons within each shell, then within each subshell, and then with each type of orbital that lies within each subshell, that is shaped by the amount of energy it has. I think what would help the viewer is explaining how external energy sources (light, heat, etc) that strike the atom can cause a change in the energy of each electron, and how that could then cause it to jump to a new shell, subshell, or change the type of orbit it has as the energy level increases. That would clarify to the viewer exactly what is happening.
This should be the way where quantum.should be taught😍Thank you! YOU MAKE ME UNDERSTOOD THE CONCEPT INSTEAD OF JUST memorizing like others do.
I was just completely frustrated between shells and sub shells and orbitals......I even completely checked the whole internet but didnt get what I needed but then I remembered khan academy just came here and now I am co.peltely cleared
I love the way he says orbital.
THANK YOU SO MUCHHHH I've spent my entire morning trying to understand what a shell is and no one put it like this. I don't know why this clicked for me but it did so thanks.
After watching hundreds of videos ...I found this one best
This cleared so many things up I was able to figure out what my school’s notes were saying after watching this vid tysm
I was feeling super stressed and found this video
But as soon as I heard his voice I was like
“Ahhhhh this will make sense, I’m safe here”
Masterfully explained. Thank you for producing such a wealth of useful learning resources.
Gosh finally a good video.
Bless you man, u really taught this concept the best
You are great .....I got the visualization ...which is was finding from a month 🙏🙏
Thank you. I needed a basic start and this helped me. Wikipedia didn't work this time for me.
watched a video of you after 6 years. Last video I watched was Differential Equations. I passed the exam thanks to you. Oh I missed your voice lol
You cleared my all concepts about atom thank you sir
Thanks, electrons as shells make sense. Have you noticed how the earth is constructed? An inner core, outer core, magma, each rotating in the opposite direction from it's neighbors, kicking out electromagnetic bands. Like a pattern of the atom.
Thankyou very much for this explanation. You taught everything on point. Again thankyou so much for making me understand this.
Oh my god its so intuitive now❤❤❤
Wow you have taught great ...اعلی بہت
Thanks a lot sir. You made me understand what I couldn't undertand through my book.
Well, Mr. Khan, you're doing a fine job with drawing the orbital elipticals as far as conveying what you're talking about, the picture's clear.
Thank you so much for such a great explanation on this topic! It was very helpful! :)
i was studying chemistry from unacademy but i was so confused in these terms.............thanks for this..........
now its all clear..........keep making such short videos they r so helpful................love love
Same here i am also at plus
@@satyamsoni1004 from whom>????
@@vartikashukla4693 I am at emerge batch 2.0 for 2022 jee and I was studying Inorganic chem by Piyush Maheshwari sir...
Suggest me some good teachers of inorganic chem. If u know any...:-)
Very helpful, thank you!
fantastic explanation
Thank you khan academy . For making this as simple . I am truly saying i am 12 th but I am cleared with this concept just now because of u❤️
Thank! Makes sense now visually
THANK YOU SO MUCH SAL. YOU ARE ALWAYS HELPING ME OUT
Oh I absoultely loved your way of teaching 😍
For those who don’t know.... there is a summary in the description
The circular path around the nucleus where the probability of finding an electron called shell
Thanks a lot. It was really hard for me to understand this in 3d.
Thanks for best explanation
PLEASE CLEAR MY DOUBT...
a. Just as you told , it's just the approximation or probability of an electron to be found , so can the electrons revolve in BETWEEN the sub shells too.. like in between 2s and 2p or 2p and 2d ???...
b. if we pump more energy to the electron , u told it can shift its orbit , then will not the electronic configuration get distrupted?
sorry if it's late. There is some overlap between these orbitals. This means that an electron can be found in regions that are influenced by both the 2s and 2p orbitals. While there is some overlap between 2s and 2p orbitals, this does not change the total electron capacity of the subshells
very helpful, thanks so much
Great ... we want more teacher like you .. 🥰😍
Very helpful video for me and very interesting
This guy has taught be so much
Ah, these chemistry videos gives me nostalgy from 2015 when I was preparing of college chemistry courses :)
excellently explained, really appreciate the support!
brother u make me cry you are great teacher
:)
i like this guy it sort of makes sense
Sir..I just miss the previous sound of khan sir who was there in 'light' chapter..the sound was so pleased to our ears and understandable too..will u be back with your sound..? All over your vdo has no loopholes 😊😊stay blessed SIR
Happy Teacher's Day, we celebrate Teacher's day today here in India
Thank you sir! Your both presentation and explanation are very helpful and easy to understand because of your speaking style.
No
I KNOW SAL IS ONLY ONE PERSON BUT I SURE WISH HE WOULD DO ALL OF THE KHAN ACADEMY VIDEOS. NOT EVERYONE IS BORN TO BE A TEACHER. JUST SAYIN
Thank you sir. 🤘✔️
Thanks alot
Question: As you add energy, orbital zone or distance of the electron from the proton increases, rendering the strong nuclear force flux weaker on the electrons? Which explain why for some reactions to take place quicker, you heat them up. By the same token, if you chill the atoms/molecules, the proton-electron distance decreases(nuclear force flux is stronger); therefore, the reaction is harder to take place. Am I on the right track?
Ya
What kind of dumbbells are chemists working out with? Lol
Can someone explain to me the mechanism that causes two similarly charged particles to repel and two particles with different charges to attract? What is going on between the two particles?
when there is a lone pair ( a pair that doesn’t have a bond with a new element) they are content and not looking for any new electrons from a new element, as they’re already paired with one another.
so H2O, for example oxygen has 6 electrons in its second shell which means its looking for 2 new electrons (by which the hydrogens will give one electron each to oxygen) this means there are two pairs of oxygen which repel the other electrons away because they arnt looking for electrons. This is actually why water forms a “bent” shape, because the hydrogen is closer, and has a stronger attraction to oxygen, while the two lone pairs at the back have a weaker - causing it to have that polarity effect.
A video on a rundown of the lewis dot structure may help.
Hope this helped.
@@Ethan-Schmidt I understand their description of what occurs. It explains nothing about what is occurring. It also implies sentience/consciousness of the particles, which is ridiculous. These particles do not think. There must be some mechanism that causes them to attract and repel, yet none has ever been provided. We are just supposed to take the description as truth on faith and think no further on the matter.
@@wesbaumguardner8829 iv only been studying chemistry for 3 months so i’m not sure how accurate i was
@@Ethan-Schmidt It is all good. They do not even have an explanation for it. I have had college chemistry and they could never answer that question. It was just "this is what happens" or "we will leave that for a higher level class" now off to the next topic. Perhaps you might ask your teacher/professor about the mechanism that occurs between two charged particles to cause them to attract or repel and see if you can get a different result.
@@wesbaumguardner8829 hahah yeah i’ll try again on monday!
THSNK YOU I LOBR YOU
U r amazing
This video goes hard in chem class
a slight correction
if you give more energy to an electron it doesn't actually jump into the other shell it teleports to the other shell
I am very curious to learn that when and how Lebniz wakes physics with calculus by locating the time affecting certain movements of a particle ( or an object) ?
Best learning
Holy, thank you
Congratulations🎉🎉🎉🎉🎉🎉🎉
6 million+ subscribers
I passed my 10 th grade and now this time is of learning deep chemistry .... as my teacher taught me that electrons are in orbits but they never taught that orbit is just a imaginary path way 😔....thanku so much 🙏
Might be a dumb question but with the dumbbell orbital, how are the electrons able to pass so close to the nucleus?
Sir please make a video to explain the structures of f orbitals.
Thank you for the video. I see many many videos on these topics, but the question persist : why electron do not end on proton ? Electrons are on shells BUT electromagnetism exist, is very strong, 10*37 more than gravity, and act on electron and proton. So WHY electron do not end on proton ?
ANSWER IS MISSING.
Greattt
Where we can get his more videos?
What I don’t understand is the overlapping, like so all the electrons in the 2p are always in the sphere of 2s, so like how does that work
And like so is 2s the whole sphere or is it a large sphere with a small sphere cut out of inside it, like will electrons in the 2s enter the space of 1s?
Like do I imagine a small tennis ball inside a basketball, and so will electrons in the 2s ever enter the tennis ball or are they gonna be just inside the volume of basketball but minus the small tennis ball inside??
how calculate an atom's energy from its electron configuration?
Great info. Well explained. What program is being used to draw?
Okay but can pls someone send the link for that 2nd video i can´t find it anywhere
Neat
So if opposite charges attract, then why don't the electrons just stick to the protons?
How can I find next video 😢
Oh interesting
That p-orbital, if it's in dumbel direction? See nucleus is the centre of that orbital which means that electron will go by that path, but won't it get attracted by nucleus then?........
@CytochromeS nah, i got it sorted out...that dumbell shape is actually away from center, i mean it doesn't pass the center....it's just wrong illustration!🤦♂️.......
What is the program you use to draw in?
is this is the voice of SAL KHAN
5M subs and 6,125 views. Aight.
Electrons actually stay in orbital, not in orbit. Orbit is a rough idea. According to Quantum Model electrons stay in orbital. So how they rotate in orbital and the shape of orbital is one of the most critical thing to say. In most cases when we talk about orbit or orbital we simply draw some circle to describe the path of electrons, this is somehow a great wrong. If you take 110 electrons you see only 10 electrons rotate in circular orbital and 100 of them rotate in complex shaped orbitals like dumble shaped or double dumble shaped or more complex shaped. So you need a clear idea about orbital if you actually want to know and do something in chemistry.
Hey, what do you use to draw these videos?
Why opposite charge attract to each other please anyone have idea then please please give the answer please please🙏🙏🙏🙏 please
to complete the outer shell and keep the atom more stable.
ugh this always confuses me
I’m paying for an education but learning from him. He has changed my entire life 🙏🏼🥹🥹🥹❤️
electron is a point charge then why WHY WHY AN ATOM DOES NOT HAVE PERMANENT DIPOLE MOMENT!???????
I love you maaaan
math antics is more helpful than khan math
100 % correct
Why can't lithium form 7 bonds to achieve a stable outer shell?
what does giving energy even mean tho