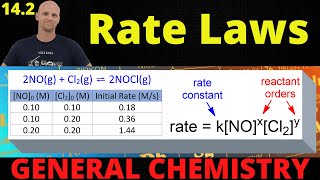
Ka and The pH of weak acids - Real Chemsitry
Вставка
- Опубліковано 9 лют 2025
- To determine the pH of a strong acid see:
• The pH of strong acids...
For an intro to equilibrium calculations and ice tables see:
• ICE tables and equilib...
In this video we will determine the pH of a weak acid. This process involves 1) Determine the equilibrium concentration of H+ through an ice table 2) taking the negative log of this concentration.
The ICE table must be used because weak acids do NOT fully dissociate. So if the concentration of a weak acid is 1.0 M, the concentration of H+ ions will typically be much less than 1.0M. To determine the exact concentration, we must use Ka. The equilibrium constant for acids.