Hexagonal Closed Packed Structure 3D Animation | Hexagonal Solid State | Hexagonal Crystal structure
Вставка
- Опубліковано 31 лип 2015
- Closest-Packed Structures
Efficient Packing of Balls
Suppose you are given a large number of tennis balls and asked to pack them together in the most efficient fashion. What is the most efficient packing strategy? One could toss all the balls together in a box and shack the box to induce the balls to settle. The resulting packing of the balls is called a random closest-packed structure. Not surprisingly it is not the most efficient way to pack the tennis balls.
Although there are a variety of factors that influence how atoms pack together in crystals, atoms generally seek the most efficient packing structure in order to maximumize intermolecular attractions. Metals provide the simplest packing case, because these atoms can generally be regarded as uniform spheres.
The two most efficient packing arrangements are the hexagonal closest-packed structure (hcp) and the cubic closest-packed structure (ccp). This exercise focuses on the hexagonal closest-packed structure, and the next exercise deals with the cubic closest-packed structure.
In a crystal the atoms are arranged in a regular repeating pattern. The smallest repeating unit is called the unit cell. The entire structure can be reconstructed from knowledge of the unit cell. The unit cell is characterized by three lengths and three angles. The quantities a and b are the lengths of the sides of the base of the cell and γ is the angle between these two sides. The quantity c is the height of the unit cell. The angles α and β describe the angles between the base and the vertical sides of the unit cell.
In the hexagonal closest-packed structure, a = b = 2r and c = 4(2/3)1/2 r, where r is the atomic radius of the atom. The sides of the unit cell are perpendicular to the base, thus α = β = 90o. The base has a diamond (hexagonal) shape corresponding with γ = 120o.
How might one characterize the efficiency of the packing of atoms in a crystal?
The volume of the unit cell is readily calculated from knowledge of a, b, c, α, β, and γ. The volume of the hexagonal unit cell, which described the hexagonal closest-packed structure, is V = 8(2)1/2 r3. The volume of an individual atom is Va = 4 π r3/3 and there are two atoms in the unit cell for the hexagonal closest-packed structure, thus the volume occupied by the atoms is 2 Va = 8 π r3/3.
The packing efficiency, f, is the fraction of the volume of the unit cell actually occupied by atoms. For the hexagonal closest-packed structure f = π/(18)1/2 = 74.05%. The cubic closest-packed structure has the same packing efficiency, and this value is the highest efficiency that can be achieved.
Hexagonal Closest-Packed Structure
The virtual reality display illustrates the packing of atoms in the cubic closest-packed structure. This display requires Java3D. If the display is not visible, consult the Java3D FAQ. Drag with the left mouse button to rotate, the center button to zoom, and the right button to move the object.
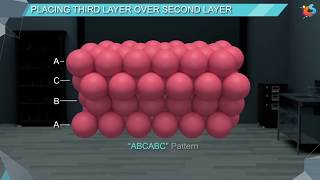
Follow the suggested steps to visualized the structure, which consist of a 4x4x4 array of 64 atoms. All of the atoms are identical; however, the atoms have been colored red and green to illustrate which rows have an identical position in the xy plane. The layers of atoms in the hexagonal closest-packed structure follow an ABABAB pattern.
Use the "+" button to start adding atoms in the first layer. Before adding each atom, think about the best location for each atom to achieve the most efficient packing (there are multiple positions). Pay special attention to how adjacent rows are positioned.
After the first layer is complete, use the "+" button to add atoms to the second layer. Before adding the first atom, think about the best position in which to place the atom.
After the second layer is complete, use the "+" button to add atoms to the third layer. Before adding the first atom, carefully examine the completed layers. Notice that there are two different positions to place the first atom in the third layer. In the hexagonal closest-packed structure, the third layer lies directly above the first layer.
Remove all atoms. Display the unit cell and the lattice positions. (Each atoms is centered on a lattice position.) Add atoms to each layer and observe the arrangement of atoms in the unit cell.








Finally I understand this topic
Tears of joy😭😭
Yes bro🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺🥺me also
these 5 min taught me what my teacher taught for a week
True
@@realkarthiknair so true
@@arnab1804 yeah
Are yeah animation hai samaj me aya teacher thori na animate karke dikha sakta to time to lagega hi na
Soo true
finally understanding the diffrent voids - was always hard to imagine
Fabian Zills rydrxyrxrxexe
@Reena Verma aap sehi bola ...
@Reena Verma ohh! How!
Amazing video, hats off the creator! You have really made our lives easier 🙏🏻
Out standing. Finally understood Nice vid
Finally, I got it
Massive respect for this guy👏
It is difficult to imagine for me how do you made animation
Probably done in a 3d modeling software, like blender for example
Too easy
It's relatively easy to make animation Because we have good theory but scientist who imagined this for First is of God level
This video gave a clear description of what i learned.
A much needed video... really beautifully explained... thanks a lot
Awesome this video entirely cleared my doubt
Its amazing , thankyou for making it so clear
Really helps me...good way of explaining by visualizing
Underrated video
I read this chapter minimum 3 times but I can understand this chapter so thank you so much for this.
Very nicely depicted. This helped me a lot.
Very Nice, It helps to learn solid states more easily, and helps to clear our concepts forever.
It is almost impossible to understand through book, and specially in online classes.
Thanks a lot for such a beautiful animation
Thank you so much dude! The vid was really very very helpful.
beautiful illustration
This makes study easy. This video is wonderful.
Thank you so much!
Thank you do much. May god bless you. Peace
Teacher took around 2 hr to explain this but I still had doubts which this video solved in just 5 mins...👍
Wonderful video with beautiful animation and amazing explanation
thank you very much
ITS WONDERFUL I LEARNT FROM IT A LOT
Thank you very much. It was really very helpful and was very easy to understand.
Very beautiful conceptual video
Thank you so much 💖
Thanks teacher.
Very helpful to understand void ✌
Was very helpful! Thank you so much!
very nice, it clear doubt regarding cn no. which is very hard to fell in paper.. thankyou for making this type of educational animation..👍👍👍👍👍👍🏼
this was needed!! SERIOUSLY NEEDED!!!!
Must watch video of solid state... really very helpful..ua-cam.com/video/VsctHFd9zsw/v-deo.html
thanx very much it was tough to imagine
I understanded clearly... thanks
Woah ..Thanks a lot...
Thank you! Now i'm able to understand it.
😎😎
Thank you so much 🙏🏻☺️
This makes study easy 👌 really very nice animation
Thank you so much
Thank you soo much.. now got it!!!
Best video ever.
Visually representation is always best way to understand
Superb animation and explanation 👍
Love you sir very hard working to students
Thank you India, very helpful.
Best ever animation
Perfect video to understand
this is very helpful!!!!!!
Thanks a lot.. It's wonderful
thank you sooooooo much
VERY GOOD INDEED! thanks.
Great!
This is so good
Amazing animated video, keep going
Very good demonstration
Understood easily with animation!
thank u soo much
Finally i understand this topic 😁🥰
Thank you
superb
Nice visualisation and explanation... 😍😍😍😍😍😍😍😍😍😍
Great video
Well explained thank you very much 👏👏👏🙏🙏🙏🤝🤝
Nice imagination thank you so much
It is so helpful for me, Thanks
Best tutorial ever ♥♥♥♥♥ Thanks..........
thanks for clear my doubt...
Very helpful video.. Thanks a lot👍
ooohhh
Now I'm able to understand
really nice.
thank you.
Now I clearly understood that
SOLID"STATION" .🙆💎🎓
So good teaching 🙏🙏🙏
wonderful
Haahh!! Thanks a lot mam!!😭♥️
Its very helpfull in visualization and that is needed in solid state chapter......thanks
Must watch video of solid state... really very helpful..ua-cam.com/video/VsctHFd9zsw/v-deo.html
Thank you so much 😍
I understand it correctly 😇😇
good content 😍
Excellent
very helpful
Good plz upload more videos thank you so much
very very easy to understand voids thanks a lot 🎉😊😊😊
Really good
Superb
Really awesome 😊😊😊👍👍👏👏
Thanks a lot now my concept of hexagonal closed packing is fully cleared
Nice explained
Very good explanation at all!....got concept now!
Best explanation ever
It is so helpful
👍👍 thanks sir
Good Animation
Thanks
Sooo thank you..
I really wanna to say thanks to the creator of this animation it's amazing and made learning easy
Thank u
Good animation ....
Very good