What is Resonance -Understanding Orgo Resonance Structures Vid 1 by Leah Fisch
Вставка
- Опубліковано 8 лют 2025
- leah4sci.com/Re... Presents: What Is Resonance? A breakdown of resonance hybrid, resonance intermediates and curved arrow representations.
Need help with orgo? Download my free guide '10 Secrets to Acing Organic Chemistry' HERE: leah4sci.com/or...
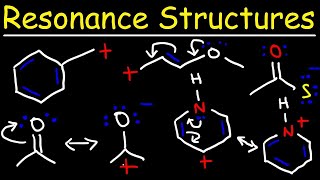
Video 1 in the Resonance series introduces the concept of resonance structures to help you understand when and how to use them in your organic chemistry coursework.
Resources mentioned in this video:
Lewis Structures Video: leah4sci.com/or...
Formal Charge Shortcut: leah4sci.com/fo...
Catch the entire series along with the Resonance Guide and Practice Quiz on my website: leah4sci.com/re...
This series requires quick formal charge calculations which you can learn here: leah4sci.com/formal-charge-formula-and-shortcut/
For even more in-depth review including practice problems and explanations, check out my online membership site: studyhall.leah4...
For private online tutoring visit my website: leah4sci.com/or...








Oh.. god, I never thought Organic chemistry could make sense... Thanks a ton.
Organic Chemistry is fun! You're welcome :)
Thank you so much, I don't know how I would get through my class without these tutorials!!!
You're welcome! Glad they help!
I am a fifth year clinical pharmacy student. When I was at the first year i didn't use to like organic chemistry because was very difficult in the peepers and board but you managed to make it so easy and enjoyable I wish all the teachers were like you!!!
Yay! So happy I could help!
Your videos are so helpful. I'm a senior Chemistry major and I've recommended your videos to so many of my classmates. Thank you so much for making these!
You're so welcome, I'm happy to help! And thanks for recommending me!
Leah this series is beyond helpful! Thank you so much! I'm prepping for the MCAT, so I'm always studying and have been watching all of your videos... It's gotten to the point where my husband comes home and says "Is Leah here?" lol! Thanks again!
lol, that's great. Hope it all went well for you!
you are literally saving me in OCHEM, i appreciate you so so much
Wow, so glad to hear this! You're very very welcome!
THANK YOU SO MUCH LEAH ! YOU'RE THE BEST TEACHER FOR OC ON ENTIRE UA-cam......i was confused if two electrons were moving or just one you cleared it right away others dont cear such basic douts which drive students nuts sometimes lol
Happy to help and you are so very welcome!!!!
I've been struggling with this topic for a while now and I finally understand it now! You literally just made me have an "a-ha" moment, thank you!
So glad I could help! Yay for 'a-ha' moments!
You are seriously the best! :) What would I ever do without you??
Aww shucks, thanks!
Wow!!! I just saw your 97 videos on orgo. Awesome Awesome. God bless your soul prof. where were you and UA-cam when I first took this course. You make it really simple. Very easy. Thank you Prof.
You are so very welcome, happy to help!
YOU SAVED MY YEAR THANK YOU 😭😭
You are so welcome!
Honestly! You are the only female teacher of Chemistry that I can understand. Thank you very much
you're welcome
I love all of your videos! I wish my professors had half of the teaching abilities that you have, it would make my life so much easier!
Glad you enjoy them. Thank you for your kind words! :)
wow, thank you Leah, this is awesome! I can't wait to check out the rest of the materials on this. makes sense now!
You're so very welcome, glad you enjoyed it!
crystall clear!thanks miss leah!!
You're welcome!
Hi Leah, I am a fun learner, though my major is not this, I started to explore deep with your videos, missed a one like you in my school days....Thanks
You're so welcome, happy to share!
this was so helpful.. I feel so much more comfortable with resonance
You're very welcome, happy to help!
Very clear explanation, Colors helped a lot. Thank you
You're very welcome!
Thanks. This material didn't make sense in class but you made so much more sense to me. I'll be watching the next vids :)
yay, so glad to clear it up for you!
only video with good examples and proper explanations!
Glad I could help!
This video has also proven to be helpful, thank you so much!!!
You're very welcome :)
For the elonate why is the charge on carbon 1 ,I’m getting a bit lost since formal charge = group number - number of covalent bonds - number of electrons in lone pair which is typically 4 1-2 if I’m not mistaken
I'm not sure which carbon atom you're referring to in the drawing, but if you'd like to review my shortcut for calculating formal charge, make sure to see my tutorial at Leah4sci.com/formal
You're a blessing!! :) Thank you!
You're welcome, happy to help!
You have an amazing voice.
Thank you
so good yes! no certain lucky oxygen gives two electrons to nitrogen, it is because all oxygen are equal, so everyone have the chances to give electron and the pai bound is hanging over the entire molecule because there are some free electrons.
great professor many thanks
You're welcome!
wonderful presentation. A++ :)
Thanks! Glad you liked it
In physics, resonance is a phenomenon in which a vibrating system or external force drives another system to oscillate with greater amplitude at specific frequencies
Thanks, but we are talking about organic chemistry
thank you a million
you're welcome!
solute to you..... incredible teaching
Haha nice pun
lol, thanks so much!
Thank you!
You're welcome!
At 4:17 you said that carbon will have a negative charge, but wouldn't that violate the octect rule?
The molecule at 4:17 does not have a carbon atom. This is the example of nitrate. Every atom in each of my resonance structures is following the octet rule. There are no violations.
Love from Bangladesh. Thanks a lot mem 💖💖
you're welcome!
Why cant all professors be like you?
and most of them talk to "themselves so polite
Aww thanks!
Awesome 👍
Thanks 🤗
you are the best in the business professor thank you
You're so welcome!
Lovin' 'Leah4Sci! 😛#newplaylist #allldayev'ryday
I'm glad you are enjoying the channel!
Thanks.
You're welcome!
you are the best
thanks so much!
Thankssss
you're very welcome!
Is formal charge the same as the partial charge?
They're very very similar. Partial charges could be attained through resonance, but formal charge is speaking of something very specific to a single atom, as drawn in a particular Lewis structure. For my formal charge formula and shortcut, make sure to read my tutorial at Leah4sci.com/Formal
@@Leah4sci Thank you very much, your video gave me a slight hope to my organic chemistry 2 lesson. It's little bit hard because we haven't learn the organic chemistry 1, but i am trying to catch up by watching your video ❣️
I thought that resonance did NOT mean that electrons were "constantly moving back and forth, back and forth". Resonance = electron democratization, and shows relationships, not actual movement.
I'm not sure I understand, what is 'democratization?'
Without getting too technical (advanced physics) the simple way to understand resonance is to think of the electrons moving or 'resonating' back and forth
FYI the rhinocerus is from Bruice not your student
I'm not familiar with Bruice, are you saying this is the source of the rhino mnemonic?
p323 in the latest 8th edition@@Leah4sci "The following analogy illustrates the difference between resonance contributors and the resonance
hybrid. Imagine that you are trying to describe to a friend what a rhinoceros looks like. You might
tell your friend that a rhinoceros looks like a cross between a unicorn and a dragon. Like resonance
contributors, the unicorn and the dragon do not really exist. Furthermore, like resonance contribu-
tors, they are not in equilibrium: a rhinoceros does not change back and forth between the two
forms, looking like a unicorn one minute and a dragon the next. The unicorn and dragon are simply
ways to describe what the actual animal-the rhinoceros-looks like. Resonance contributors, like
unicorns and dragons, are imaginary. Only the resonance hybrid, like the rhinoceros, is real."
So resonance is actually fluidity. Fluidity creates an intramolecular communication network which inturn creates molecular self awareness. If there is fluidity then there are no still electrons, all electrons are moving with each pair of electrons having a turn to go in to form the double bond.
Yes, resonance is the idea that certain electrons can be delocalized and are allowed more freedom of movement across a molecule.
Why does Nitrogen have a positive charge?
In the case of the nitrate ion, the nitrogen has four bound electrons and no lone pairs of electrons. According to the equation for formal charge, that's 5 valence electrons minus 4 bound electrons equals a formal charge of +1.
For more on formal charge, make sure to see my tutorial at Leah4sci.com/formal
I got question: How can I make synthesis of some molecule because of resonance? If all molecules resonate then which one is the correct structure as end-product of my intended compound? Or even - example aspirine molecule - if Im looking to wikipedia or search in google then I found many different versions or my intended compound so how can I something do and make a synthesis if these molecules are resonate all the time?????????? Kind Regards.
I'm sorry, but I don't offer tutoring through UA-cam comments. For help with this and more, I recommend joining the organic chemistry study hall. Full details: leah4sci.com/join
i have a doubt
in that cyclohexane example
how can the carbon at the bottom have a lone pair
the octet is not complete
at which point in the video?
Leah is it possible to upload vedios with english subtitles..
All my videos have English captions
roses are red. violets are blue. nobody teaches better than you. thanks :)
aweee! Thank you and you're welcome :)
i want more videos
You can use leah4sci.com/syllabus to find all of my orgo resources
omg dinasour + unicorn = rhinocerous
biology i tell you :)
genius
Glad you liked it!
Dinosaur+unicorn=🦏. 😆😂🤣
Right!! 😀