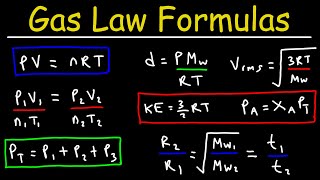
Solving the Ideal Gas Law for Temperatures (T)
Вставка
- Опубліковано 2 жов 2024
- In this video we’ll work a practice problem for the Ideal Gas Law, PV=nRT. For this problem you can rearrange the equation to get T by itself to start with or just plug in values and solve for T.
Join this channel to get full access to Dr. B's chemistry guides:
/ @wbreslyn
Gas Laws Playlist: • Gas Laws & KMT
1. Given values:
Pressure (P) = 2.0 atm
Volume (V) = 3.0 L
Number of moles (n) = 0.5 moles
Ideal gas constant (R) = 0.0821 L·atm/(mol·K)
2. Use the ideal gas law equation: T = PV / (nR)
3. Plug in the values: T = (2.0 atm * 3.0 L) / (0.5 mol * 0.0821 L·atm/(mol·K))
4. Calculate: T = 6.0 / 0.04105
5. T = 146 K
Other Videos about the Gas Laws:
• Ideal Gas Law: • Ideal Gas Law: Example...
• Real vs Ideal Gases: • Real vs Ideal Gases
• When to Use the Ideal Gas Law: • When to use the Combin...