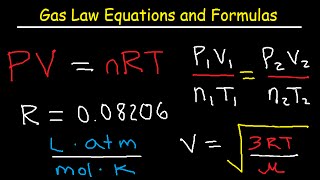
5 Ideal Gas Law Experiments - PV=nRT or PV=NkT
Вставка
- Опубліковано 13 бер 2016
- The ideal gas law may at first seem very abstract but it’s surprisingly easy to demonstrate the the various relationships between the elements. This video gives 5 simple experiments that you can do at home or in the classroom that doesn’t require specialized lab equipment.
Blog Link:
youcanscienceit.com/2016/03/1...
Linkography:
• The Sci Guys: Science ...
• The Sci Guys: Science ...
• The Sci Guys: Science ...
• Testing the Ideal Gas ...
• Gas Law Experiments
• Boyle's Law and Marshm...







![Master the Ideal Gas Law in Chemistry - A Step-by-Step Guide - [1-5-10]](/img/n.gif)
Great Job! This has a fun but smart vibe. I look forward to more!!!
Great video this was a huge help for my school science project! You just earned a sub :)))
Thank you for this video! I think I might do the marshmallow one for school :D hope you upload more videos like this, they have great potential!
It helped a lot. I have a test tomorrow and had this as a doubt but now it's all clear and it really helped a lottt.
Thank u sir
Wow !that's amazing and really helpful
You're so helpful!
Gud experiments for ideal gas nd all rightly understand this experiments thanks professors to guide the students truly
nice man, keep them coming :)
Great ideas! While I was aware of most, I never thought to use the duster to cool a balloon, and will definitely be using that next year with Gas Laws. Neat how duster is used similarly in the 10 Cloverfield Lane movie that just came out!
+MrLundScience Thanks. Try and find an air duster without the bitterant. It's really unpleasant.
Love it!
Really interesting demos! That being said, I'm concerned that you're advocating for some unsafe experimentation practices/prepping for experiments (e.g. with the liquid air, drill). Would strongly encourage adding notes on how to do those set ups safely and putting a note on the video.
It's too bad that you stopped making content. I get it though, but I just discovered your channel and like you're curiosity and how you go about satisfying it.
you are great as other channels just explain you do and explain
you are so cool, you are cooler than that water bottle before your hands warmed it up
that was amazing it really helped me understand the law lol
This is so cool
Bohat allaw welldone
That's what I'm searching or what I want to understand... Thanks a lot sir.....🎂pls accept this cake☺️
i have a chemistry project due at midnight guys help...
fuck he changed the deadline thank fuck
me rn
Same - we are teaching a whole unit ourselves. So basically a seminar
Can we use ice???
Excellence ...
This made me laugh so much! LOL
Once you are spraying long enough the N changes as the pressure changes. The T is due to phase change of liquid to gas
Interesting...
It doesn't bother you that you actually changing N in some experiments?
0:54 the music sounds like someone is popping a balloon in the background
I see some other folks have raised similar points as what I list here that N is changing in some of these experiments so the explanations are not holding true.
Experiment #2:
The explanation of why the container get cold is not completely true. The video explains that P and T has direct correlation but that is not completely true because N is changing. The main reason for the change in T is due to phase change of liquid to gas which absorbs the heat.
Experiment #4:
Marshmallow is not a ideal Gas so I wouldn't really like to see P*V = N*R*T for this one, I'll give you a pass for this one as you are trying to give a good visual.
Experiment 5:
The N is changed so again you can't say T and P have direct correlation and more over you are adding Energy to the system by your pump. As a matter of fact, in order to double the pressure, you have to double N so T shouldn't change if you didn't insert energy to the system by your arm.
The reason for the GAS getting warm here is that you are using a Hand pump and your mechanical energy (from your arm movement F*D) is converting to heat.
"Marshmallow is not an ideal Gas"
says you, buddy! I'm kidding, great addition to the video.
But.....I have a question sir...Gases inside balloon or bottle or sprayer are real gases....Then how they are obeying PV = nRT .We have taught that only ideal gase(hypothetical gas which doesn't exist) follow this ideal gas equation.....
Pls replay sir....
-your fan (from this video)☺️
I'm not an expert, I'm learning this just like you are, but here's my understanding of it.
I think with real gases, you replace R (the "universal" gas constant that's not really universal) with the constant corresponding to the gas you're using.
And even if you aren't using ideal gases, all real gases follow the principles to an extent (to a second approximation), it's just that the ideal gas laws don't take into consideration some other factors such as intermolecular forces that act between the gas particles.
So real gases won't follow the formula *to the t*, however, the general relationship between P, V, T, n, and the gas constant will still hold.
Someone please correct me if I'm wrong, I would love to learn more on this!
@@jeewoo7195 Every physical model is an aproximation. If the molar volume of the gas is low enough, and its temperature far from liquification, experiment shows that PV=nRT is pretty precise. There are some more complicated models for special conditions like high molar volume, such as you said.
mafynetti
Yang kesini gara gara tugas gas termo wkkww
LOL