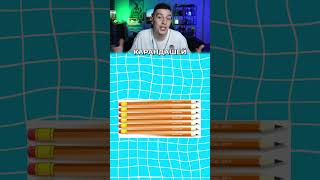
Pure sulphuric acid is a liquid of 1.84 density. What volume of it would be required to prepare...
Вставка
- Опубліковано 5 лют 2025
- Hello everyone, in this video, we'll be solving the question below;
Pure sulphuric acid is a liquid of 1.84 density. What volume of it would be required to prepare 250cm^3 of 0.2M solution? (H = 1, S = 32, O = 16)
telegram channel: t.me/adeyanju_mercy
density, volume, how to find density, density and volume, density mass and volume, density formula, how to calculate density, stoichiometry, stoichiometry problems, stoichiometry mole to mole, stoichiometry moles to grams, stoichiometry problem, stoichiometry tutorial, solution stoichiometry problems, stoichiometry chemistry, mass volume relationship, mass, volume, mass volume relationship examples, mass volume, mass volume stoichiometry, mass-volume relationship