How to Draw Lewis Structures, The Octet Rule and Exceptions | Study Chemistry With Us
Вставка
- Опубліковано 24 бер 2020
- I'll cover how to properly draw lewis structures of regular molecules and lewis structures of ions. We will also go over what the octet rule is and all the exceptions that go along with this. Plus we will talk about the valence electrons you will need to memorize for your next chemistry exam.
📝 DOWNLOAD THE STUDY PLAN
melissa.help/studyplan
📗 FREE CHEMISTRY SURVIVAL GUIDE
melissa.help/freechemguide
📘 FREE ORGANIC CHEMISTRY SURVIVAL GUIDE
melissa.help/freeochemguide
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
chemmunity.info/youtube
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
melissamaribel.com/
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
melissa.help/thermonotes
📗 Acids and Bases Guide
melissa.help/acidbase1notes
📘 Naming Compounds and Acids Guide
melissa.help/namingnotes
📙 Dimensional Analysis, Significant Figures, and Density Guide
melissa.help/sigfignotes
📕 Gas Laws Guide
melissa.help/gaslawsnotes
📗 Stoichiometry Guide
melissa.help/stoichnotes
📘 Redox Reactions Guide
melissa.help/redoxnotes
📙 Molarity Guide
melissa.help/molaritynotes
📕 Limiting Reactants Guide
melissa.help/limreactnotes
📗 Lewis Structures Guide
melissa.help/lewisnotes
📘 Kinetics Guide
melissa.help/kineticsnotes
📙 Titrations Guide
melissa.help/titrations
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
melissa.help/matterguide
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
melissa.help/cheggstudy
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
melissa.help/cheggbooks
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!
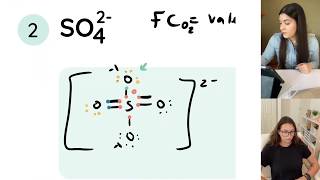








My Lewis Structures notes are here if you would like a step by step guide on everything Lewis Structure related!
You can purchase the notes here ➡️melissa.help/lewisnotes
Hello MM, ...what happens at the sides of a graphene layer related to bonding?
Thanks ❤
You are literally a life saver I don't know how I would pass chem ONLINE without your videos. Thank you so much
Literally when u don’t get something at school your furies teach u or give you the answers 😈
@@ItsKenyangirlAbby i'm her best friend lol but yes she passed with a really good grade!
I just wanted to say that I am 48 years old and I am not totally confident that I can finish my AA. I am taking Foundation of Chemistry. To my surprise, it is not just Chem, it's also Organic Chemistry and Physics. Because of life and adversity, my grades are not great. I am still working on bringing my GPA to 2.0. After 3 semesters, taking 1 to 2 classes each, my GPA is now 1.994 from 1.389. I was told by an planning advisor last year that I will NEVER make it into Medical School. This semester is off to a rocky start. But watching your video, I am actually excited. I took a quiz Friday and didn't pass it, but I still got a higher grade than I have before. I recognized most of the questions. It was only 5 of them, but if I had spent more time (more than 4 hour before the quiz was due) on watching your video and practiced, I believe that I would have passed it. I am horrible at taking test, no matter how much I studied. I have tested anxiety because of my low confidence in learning, absorbing and test taking. So today I watched a few of your videos from the beginning. I am going to do that everyday this week. I have a test due on Friday March 5th. I wish I could find the words that can express my appreciation for you and your videos. I am so scared, but I actually understand what I am learning from you. Thank you so much and I hope I don't offend you, but God bless you.
How's it going? The good points you got going are your stamina and desire to get it done. You have chosen a noble path that will only make you a better person. Keep plugging away. Don't give up trying. Many would give up but I can tell you are made of much stronger fabric. Be productive!!!!
From the other side of the globe , I will say never give up !!
Sheesh. 1.0
Never give up! I'm so proud of you, that's some great dedication!
YOU'RE THE REASON I HAVE A 98.7 IN MY FIRST CHEM CLASS IN COLLEGE & I LOVE ITT ! my favorite subject ! ❤️📚
That is awesome! Congrats 🎉
Damn yall r learning this in college?
Your doing this in college?
@@209balls yup
@@KH-ks7si you realize this topic comes back in general chemistry in college. in my first year, I've revisited so many things I've learned in high school in more depth and difficulty. you need to do it again in college just to get prereqs out of the way. you can start doing ochem and other things after you've passed gen chem.
Seriously thank you so much for these videos, I have been basically teaching myself Chem and I have learned more from your videos in one day then the entire month and a half I have been in school! Thank you!
WHAT WHAT WHAT THAT MAKES SO MUCH SENSE NOW THANK YOU SO MUCH!!!!!!!!
Girl, god bless you! I’m trying to pass my chemistry placement test and this is helping me more than I ever would imagined! Keep it up
omg i wish every teacher was like you! i feel like i can pass my chem test now😭❤️
Your free chemistry guide is a lifesaver!! Thank you for being awesome Melissa!
my teacher doesn't teach he just gives us info and makes us solve it
ikr thats why melissa maribel is a total lifesaver
oh this was totally great ! It helps me a lot to understand more about Lewis Structure. Thankyou for this.
You are my life saver!!! Thank you!!!! Our Chem professor even suggested your videos to her entire class because you make Chem so much fun and achievable. Wouldn’t be enjoying Chem if it weren’t for you and my professor. Shout out to my favorite College Chem teacher, Professor Laura ❤️
Thank you so much. I'm studying for my exam and this is really helpful. I feel confident because of you!
We have a substitute in our chemistry class right now and let me just say your videos have been so helpful for me, thank you!
gah. so grateful I found you. I have a university professor who doesn't even lecture, just hands out readings, and is like enjoy--this has helped me SO much. Thank you.
Professor Melissa, thank you so much. Huge huge help. I was struggling understanding the exceptions with B and Be, also about the lone pairs and your student asked the precise question in my head (we already have to respect the total number of valence electrons involved and not go over them). Thanks a million!
Thanks for your videos! I appreciate when you point to things and the fact that I can see your face when you talk. I'm in an online chemistry course, where pointing to things and seeing a face is not a luxury! Haha! Between you and a couple other UA-camrs, I am confident I'll pass this class. Thanks so much!
YOU ARE SUCH A QUEEN YOU HELPED ME UNDERSTAND THIS SO MUCH
Love these videos, so informative and clear!
This was very helpful, I really prefer these longer videos that go through so many examples!
Thank you so much for explaining everything is such a way that is very easy to understand! Your videos have help me so much.
OMG WHY DIDN'T I FIND THIS EARLIER!! SUCH A LIFE SAVER !! THANK YOU
I LOVED the in depth explanation of each example!! THANK YOU!!!
Thank you for your hard work! It really helps me to understand better!
Thank you so much for making me understand this topic!!!!! Very grateful to you
Thank you Melissa! Your explanations are so helpful and your 'can-do' attitude as well!
The best video on lewis structures I've seen! thank you!!
This was so so amazing✨✨
You explained it too well🥰
Thanks👍
I've been teaching chemistry for 4 years and i'd like to apply this method in my school. thank you so much
This video helped me get a perfect on my quiz! Thank you so much!!
Such an amazing teacher!!! I love these type of study sessions, I feel like I'm in a virtual small group. :)))
Literally, i am just speechless. you had just given me a long lasting concept.
thank you so much for this tutorial!!!!!. It really helps me about my chem
My senior 2022 currently soon to be a 22’ graduate Ms Melissa Is The Reason I have aceing ! my chemistry class thank you it’s not many like you !
So helpful! My first exam I did not watch your videos and got a D on the exam. Exam 2 I watched your videos on the concepts and earned a B+, A with the extra credit! Thank you, thank you thank you!!! These videos are so helpful and I am doing so much better in Chemistry now :)
Congratulations 🎉 So happy to hear you are doing so well now!
I am in Chem 1 this summer and after every lecture, I watch your videos to figure out what the hell he's talking about. Thank you for making these videos!!!
lol relatable
this is me rn... I have my final tomorrow
OHHHH My GOSHHHHHHHHHHHHHH!!!! Although I spent more than 4 hours watching other videos just to understand this chemistry concept, I still was not able to completely get it. You just made me understand the whole concept in less than 40 minutes. Thank you very much, now I can assertively jump on my quizzes.
Thank you for making it make sense!
Thank you for such an excellent explanation, it really helped!
Aghhh thank you! You make it so understandable!!!!
I really appreciate the way you explaining the lws rule
Thank you
Thank you so much, you're like my favorite Chemistry teacher.
wow your explanations are eveything!!! I am understanding at a deeper level than how my professor explains
Am preparing myself for NEET that is 2nd thoughest exam in INDIA 🇮🇳 to crack (1st is JEE) and trust me this helped me in a lot of things i had a lil doubt but this lady just clear every of them thanks for explaining seeing forward to watch more videos
Lots of love...🎉 ❤
Thank you so much, I really found it helpful
Cramming your vids the day before my chem final… You're a life saver!!!
THANK YOU SO MUCH.... I have an exam tomorrow and your videos have been helping understand better.
You are a lifesaver; keep uploading more videos.....This video helped me a lot and cleared most of my doubts.
I wish there was someone like this for accounting these videos make things do clear l
Thank you for this Melissa :D
You’re very welcome 😊
Melissa: What a knowledgeable and engaging instructor you are ! Very helpful video that clarified basic concepts. Plus...how refreshing it is to see and listen the voice of a well-versed woman among the multiple available on-line chemistry videos !
Thank you so much Clara!
at first i wasnt going to click because the video was so long but when i did i was so happy i did because this helped sooo much
thank youuuuu
my exam is tomorrow and i just finally get it. THANKS!
same
thank you so much for doing this
THANK YOU FOR THESE
Thanks so much queen! You rock.
That makes a real sense....thank you so much mam🙏 Love from India
I cannot thank you enough for this video! Unfortunately my chemistry class is online this semester and it's harder to understand explanations via email and zoom isn't an option due to my schedule. I was having so much trouble with this
Thank you so much for this!!
You’re very welcome!
your teaching style is amazing
Wow, I wish utube was around whilst I was at school. Just watched your video and got the hang of how bond's are worked out. One science teacher just threw metal rods at us lol
I did go on to manufacture fireworks, but wish I could have been able to write up what I was actually doing and why I got the beautiful colors.
Cheers, from an Australian pyro
Thank you Melissa for this video, it was very helpful. Please may l request that you make a video on mole concept, it will go a long way. Thank you so much.
I just watched this whole thing, literally. Know nothing about it, but I am interested.
Thank you very much mam💗
really i love your teaching style its good thankyou
do you take money for teaching this girl
Thank you so much!
Thank you so much! You help me a lot! TwT
For the CO2 example, why does oxygen begin with 7 valance electrons instead of 6? There are 3 lone pairs but still a bond between an extra valance electron with the carbon. 🤔
I wondered the same thing.
THANK YOU VERY MUCH❤️❤️
love it thank you
Thank you!!
Thank you thank you thank you!!!!
Hi Melissa! First off thank you for all your content - It's helping me a ton in my 8week summer session of college gen chem.
I have a question about the final two structures.
When I drew my structure for ClO4-, I tended to want to draw my Cl atom with double bonds for 3 out of 4 of the Oxygen atoms and a single bond to the 4th Oxygen atom. The single electron hanging around by itself on the outside of Oxygen kind of made sense in my mind because the whole molecule in the end was supposed to be negatively charged. My main focus was making sure that the central Cl atom had 7 electrons attached to it.
I'm not understanding how its okay to leave a central Cl with only 4 electrons when it's supposed to have 7.
I hope that wasn't too confusing of an explanation. Is it like a law of physics no-no to have a single electron hanging out by itself on an exterior element or something?
Thanks for any insight!
Thanks!!
Thank you so much
THANK YOU.
Thank you so much ma'am...
I was crying this morning not knowing how I was going to write my test and then I found your videos ❤thank you very much
whaaaa thankyousomuch now i understand na
A week into grade 12 Chem... I just found my new tutor, yay🤪
You're great keep going
Hello, I am Tamam from Indonesia. Thank you for this video.
THANK YOU, GRABE
in 19:47 can we keep, one lone pair to carbon as well as to oxygen and keep one bond pair of hydrogen to oxygen
THANK YOU
MS.Melissa I also a chemistry tuition teacher teaching ISC 11th grade students . Your video on formal charges is excellent and very clear . It helped a lot to handle my students. Thanks a lot . Can i send my doubts. Isit possible for you to clarify. Thank you
Really helpful video! Quick question: for the ClO4- ion, shouldn't there be 3 double bonds between the Cl and O, since that would be the most appropriate in terms of formal charges?
thanks😊
how do you know how many central atoms to use? will there ever be a case where there are more than two atoms in the center? THANK YOU for these videos they are saving me right now!!
You are way too amazing! OMG I love you. LOL
I wish I could buy and send you a gift. You are an angel. Thank you.
I'm a sophomore watching this before my midterms tomorrow 👍😆
hi melissa do you have the instructional notes where you check things off for these?
I love you thank you
For the lewis structure of IOF5, why is I in the center of the lewis structure, when O is more electronegative than I? O is much closer to F than I on the periodic table. Thank you,
Thank you Melissa for your amazing dedication. Question: why does Oxygen consistently have 3 lone pairs if I t has 6 valence electrons and is bonded to an element?
Same question! Like if all 6 electrons are hanging on the outside, what's keeping it attached to the central - that's what tripped me up at least.
@@mirandabeaudry5337 EXACTLY!!
learned more in 36 mins than a whole year class someone give this mami a mf raise
I have a chem final tomorrow... you saved my grade
Hi, does it matter (in CH3COH) which lone pair electrons are moved to form teh double bond?
thanks