Quick & Easy: 5 Steps to Drawing Lewis Structures with Examples, Practice Problems, Summary, Explain
Вставка
- Опубліковано 5 січ 2018
- 🎯 Want to ace chemistry? Access the best chemistry resource at www.conquerchemistry.com/maste...
📗 Need help with chemistry? Download 12 Secrets to Acing Chemistry at conquerchemistry.com/chem-secr...
💯 If you like my teaching style and are interested in tutoring, go to www.conquerchemistry.com/onlin...
📝 Access my chemistry notes, cheat sheets, and study guides
conquerchemistry.com/store/
👉 Support me on Patreon 👈
patreon.com/conquerchemistry
💻 Check out my highly recommended chemistry resources
kit.co/ConquerChemistry
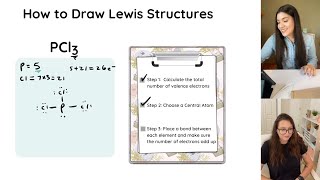
The steps are
1) Count the total number of valence electrons
2) Connect all atoms with single bonds
3) Add in lone pairs such that all atoms that want an octet has an octet
4) Count total number of electrons in lewis structure and compare it to the number you calculated in part 1
5) If the number matches up, then you're done!
6) If there are too many electrons, form double or triple bonds
7) If there aren't enough, add lone pairs on central atoms until you have the correct number of electrons
Examples include:
CH2Cl2
HCN
BrF5
By the end of this video, you'll know exactly how to draw lewis structures.







So straightforward. My teacher was a little bit vague on lewis structures and now I can just memorize a few steps and be done with it.
Thanks!
Your good! Love how you just get straight to the point. Quick and straight to the point. Perfect
straightforward amazing!!!!
This is amazing!! So helpful!
you are a great teacher... i cant thank you enough!
You are amazing and make everything so easy to understand thanks so much !!!
It's so simple. It's so easy to understand and follow. My high school chem teacher made it so much harder.
Great Teacher!
greatest video of all time
Awesome. Please make more examples
Well understood thank you very much 🙏
Amazing thank you❤
Bro your content is amazing and easy to understand
Wow you born to teach chemistry ❤
thank u this helped
Superb teacher? Sir from where u r
I love you thank you will pray for ur good health and success ❤
great work
Good. It would be perfect good if you show the Periodic Table each time where you got the variance electrons for each element
Thanks
You saved me
I get ,it was very hard at first but when I was it it makes me think easy
AMEHHHHHHZINGGGGGG
Love u
Yo, I have a question.....I understood that if we have H then its at the terminal and if C then in middle but with BRF5 we had none and you took Br in the middle so my question now is why? If we do not have C AND H, how do we decide which is gonna be in the middle?
The least electronegative atom tends to be the one in the center. We know that Fluorine is the most electronegative atom on the PT, so Br must be the central atom. Hope that helps!
hydrogen can form one bond so it can't be in the middle so it has to be terminal
Also, most atoms are bonded to a single atom. Here there are 5 F and 1 Br, so F atoms bond to central Br atom.
thank you so fucking much bro
Why does Br have 10? You loved too fast when you were trying to explain this. But thank you!