Hydrogen Bonding and Common Mistakes
Вставка
- Опубліковано 16 чер 2012
- To see all my Chemistry videos, check out
socratic.org/chemistry
Hydrogen bonding can be so confusing, and in this video we talk about some common mistakes. Hydrogen bonds are intermolecular forces between molecules. They form because one atom has a high electronegativity, so it gets a partial negative charge, and the hydrogen gets a partial positive charge.








Analogies are always imprecise. But through my years in the classroom, I've never encountered any student confusion about this issue. Because, honestly, when students are learning this for the very first time, they just need an "image" (like magnets) to make the concept more relatable. It's only people like you and me (who understand the nuances between magnetic and electrostatic forces) who worry there'd be confusion. Most 14 year-olds just think "OK, they stick together like magnets."
extremely right
so are F O N the only elements that are able to form hydrogen bond??
@@eunsoo4118 yes
Why Cl doesn't form H-bonding?
@F Rogers wait ..... Cl doesn't form hydrogen bond 🤔 really ?
But
H--Cl . . . . H---Cl . . . . . H---CL
Wrong question chlorine form hydrogen bond
you put my college professors to shame. These people have PHDs and cannot convey this knowledge the way you do. That being said, the world could use a lot more people like you and a lot less professors like mine.
how is it going learning about biomed?
BlueIceAni
this is *BIO-MED??*
I believe he has a PHD as well lol.
@@whatthehec6736 he does from MIT lmao
So true @senor
even after 8 years this guy best teacher! Anyone here in 2020?
Me
oh yeah me too
wow
me too from Pakistan 🇵🇰
Yep....
No I am not
It''s funny because my chem teacher is terrible so this guy has been teaching me all semester xD
Yeah I’ll let
Bruh Same me and my friend just subbed
Sameee
Harry Wilson so funny.....wait what was the joke again🥴
@@Lopooop216 The education system is a joke.
Hey everyone, I'm here to help. If you have any questions or just want to learn more, click on the link in the description above. It'll take you to a page where you can ask me questions.
hi do i need to memorize all elements in periodic table for this
@@itsmerhen7297 whatever ur teacher wants, but im only in gr 11 so i dont know. i never had to memorize all the elements, there are too many
Please add time
OUT-f@cking-STANDING explanation. I should just pay my tuition dollars to you.
@@lolindividual7055 Hmmmmm.....
best explanations one can find ... I am sticking to this channel for my bio class... thanks for sharing.
This is a chemistry video buddy. Not bio🤣😂
🤣
🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂🤣😂
@@Lopooop216 Im taking Honors Bio and our first unit is organic chemistry
They are related
I spent the past couple of weeks not understanding intermolecular forces/hydrogen bonding at all!! My textbook was way too complicated to understand. I can't thank you enough for making this video, I finally understand this and I am so happy!! :)
Thank you Sir that was a flawless explanation. THAT'S what I call a great video.
YOU'RE SO AMAZING, I MISSED THIS LESSON TODAY, U SAVED ME THAAAAAAAAAAAAAAANX ALOT
Coming from another teacher, you are a natural educator!! Very few people who are good at chemistry and mathematics can actually explain it so clearly, simply and thoroughly, so that it makes sense and can be visualized. It makes me sad that it is such a rare gift, had I been taught better in high school, I would've had a very different life and career. Anyway, well done and thank you so much for taking the time to do this, outstanding!
Loud and clear, and very easy to understand. Awesome, thank you.
you really put a lot of effort on this video, and i also appreciate your creativity! support and hope you can work more on chemistry! thank you!
This guy just saved me from failing in honors chem. Thanks
LOL😂
Why the hell did you take honors Chem?? That's a death wish and a half, lmao.
@@malevolentthedragonso what im taking AP chem and i still forgot
lol
this is the best video on the internet and also props to the guy for being so incredibly prepared. He must have had to think through the whole video to make those pieces of paper, and I AM SO GRATEFUL
I GREATLY appreciate this, please do more of these. They REALLY help me understand key points for my tests
Superb explanation.. crystal clear..
Remember FON! That's actually so helpful! Thank you!
Hi Mr Tyler Dewitt
I came across your Chanel and since then, I've been having A s in chemistry. I thank you not only for the time you spend preparing video but also the patience you have to explain stuff. Thanks so much
Your videos are awesome and I can always understand everything so clearly after them! Can you make one about dipoles (or do you already have one) ?
so far, he makes the best chem tutorial videos! good job and thank you for being helpful for people who are struggling with chem :D
Thank you!! I was so confused because I thought H-bonds were simply the bonds between a hydrogen and an F, O, or N atom in 1 molecule. Now I understand that a bond between an H and an F, O, or N just allows for the *potential* to hydrogen bond!
The visual and clear explanation were INCREDIBLY helpful!!!
Wow, I accidentally clicked on your profile while scrolling through some comment section and what do I see? A chemistry playlist! A week before my chemistry mock exam! Sir, thank you so much for uploading this, it literally saved my procrastinating a... bottom ; )
Best wishes!
Thanks for these videos! Im in Chem 1 and I needed some more in depth info in order to feel confident for my exam on Tuesday. I realized that Hydrogen Bonds occur between two individual molecules, and not between atoms in say a water molecule as I thought!
the whole video it looked like there was an inside joke you had going on with yourself and you'd break out into laughter any second. Other than that great vid! turned such a confusing concept into an easy one thank you :)
I have seen so many videos, but none like this one. Its amazing how great and simple you presented the concept that even my kids can understand. Please keep doing videos like this simple, simple, simple. Great job!
saving me 7 yrs later in 2019, i respect you so much. make more chem vids
I watched your video one more time, and i did understand. Thank you again.
Thank you so much for your video! It was really useful and I love the effort you've put into it.
But, one question: isn't the ethanol molecule meant to have a V-shape? I know this video is meant for beginners but I needed clarification. Would its shape not affect its ability to bond?
+Ifrah Ariff Good question! There are many ways to draw the ethanol molecule, and no way is 100% correct, because we're trying to represent a 3D object in two dimensions. However, the shape of the molecule isn't going to affect its Hydrogen-bonding ability.
But doesn't the length of the alkyl chain affect the alcohol's intermolecular bond strength, and thus its boiling point?
+Ifrah Ariff The strength of a single Hydrogen bond will always be (about) the same. If the alkyl chain is longer, the molecule is heavier and there's more to hold in place. So you might say the *total* intermolecular strength is lower, but strength of an individual hydrogen bond isn't affected by molecular size or shape.
ser. can ask you? if we have 2 or 3 oxygen molecules.? what heppen? can you show me please
Can you do a video on Polymers plz
Thank you so much for this video🙏 Your descriptions and way of phrasing things out just make it so much easier to understand then my teachers who just read off slide shows
Great TED Talk, just had the pleasure of watching it! Thanks again
Can't thank you enough. Finally! I get it now.
You're great thanks for the effort! :D
Can't thank you enough for these videos, super helpful and the best ive seen so far ! Do you have a video on how to write ionic equations in terms on precipitation/dissolving?
Easy, simple, to the point. Thanks so much for the helpful video!
COME BACK TO UA-cam PLEEEEEEAAAAAASSSSEEEEEE! WE NEED YOOOOUUU
how lucky would it be to actually have you as a teacher
I've learned this material several times, and have to keep relearning it because I was never am able to grasp the concept to where I understand and it sticks with me.
This is my first time watching your video, and you explained so much better than any of the other professors or videos has every explained it. You made it so simple but still with so much detail, I felt I genuinely understood it this time. Thank you
You make it seem so simple! Great job!
sir,can u please make some videos on organic chemistry and specially on carbon and it's bonding
I struggled with this concept for over a year...until i stumbled across ur video
thanks so much! all this time i was struggling...I finally understand it now! Thanks!:D
Betty Bear same here✌
Tyler, you're awesome! I'm 54 and learning chemistry for the first time with relation to becoming a nutritionist. If I need help I know I can rely on your videos. Thank you.
this is the best chemistry video I have ever watched, you can tell that he is passionate about the subject and spent a lot of time planning the video with all the paper and draws and smooth transitions. It was so good that within the first few minutes I had to like the video and subscribe.
IMPORTANT: point of questioning: my professor said that H-bonds can be between atoms of the same molecule (which means hydrogen bonds can be intramolecular forces) I'm not sure who is right.
Thanks to u I think that you put much effort on this vidso
Sir...please make videos on isomerism topic...
Literally been studying this for 2 weeks and you made it clear in 10min. Thank You.
Part of my test tomorrow this, and admittedly I've not revised at all - but this video was so helpful!
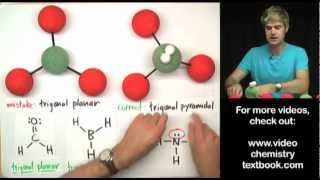
Hydrogen bonding is when Hydrogen is bonded to: Fluorine - Oxygen - Nitrogen
H- F
or
H - O
or
H - N
what year are you in
Bukhosi Hadebe
thank you so much! you just saved me from failing a chemistry test
Your videos are by far the best at explaining! I watch endless amount of chemistry videos and they all confuse me more. Thank you so much!! I just wish you made a video to help me understand how to draw Lewis structures correctly.
I am so glad I found your videos! This video was extremely helpful in learning material in my Biology 1406 class! Thank you so much and I will be watching many more videos in the future I am sure!
in 9 minutes you did what my chem professor attempts to do in 2 hours
Learning Outcomes:
1. Hydrogen bonding is intermolecular i.e. it only occurs *between* two different molecules
2. H bonding only occur with high EN elements(O,F,N)
3. common mistake: it should not be attached to any carbon
Your chem videos are SOO HELPFUL! Thanks! 👌
I'm so glad to find information conveyed SO CLEARLY
This video was super informative thank you!! (it's still relevant in 2019 btw)
kaytlyn watson damn, you'd think science would just change. Crazy
I saw all the erors in my thinhing and went OH
I went "OH" too! Together we could form a hydrogen bond xD
@@nottoday2650
Y"OH" guys crack me up. Can I also form a bond with you? We can start the best family of OHs. LOLOLO
Creeper! OH man . . .
Tyler, thank you for making this video. I was having a hard time in my bio class trying to understand hydrogen bonds, but now i feel i understand it thanks to your video. keep up the good work!
Thank you for clearly explaining and drawing the possible bond pathways!
The best chemistry teacher in the world ❤️❤️❤️❤️❤️❤️🙏🙏🙏 love from India
I THINK I LOVE U!!!!
What I truly enjoy about his videos is how he really explains in a fun way why this particular reaction must happen etc. unlike profs who just gloss over the whole thing. it made more interested in chemistry
Something so confusing has never been drilled into my head this quick. Ammmmazzing!
Thank you for making me pass Chem 100
The name is less misleading in German, where it basically is called "Wassterstoffbrückenbindung" which is German science-babble for hydrogen-bridge-bond, thus implying that the bonding is just a weaker connection between two molecules, not a strong bond between Hydrogen.
Honestly I come here everyday after lecture and search to see if you have any videos covering the material we “learned” in class. I always understand things after! Thank you so much!
You are an INCREDIBLE chemistry tutor. Absolutely phenomenal. The PatrickJMT of Chemistry! Thank you for this UA-cam channel!
Won't there be hydrogen bonds with chlorine as well? Chlorine has the same electronegativity as nitrogen.
st0nnec0ld Atomic size is important as well, and Cl and N are different sizes. So Cl has similar electronegativity to N, but only Fluorine, Nitrogen, and Oxygen can participate in Hydrogen bonding.
st0nnec0ld Atomic size is important as well, and Cl and N are different sizes. So Cl has similar electronegativity to N, but only Fluorine, Nitrogen, and Oxygen can participate in Hydrogen bonding.
Tyler Dewitt why did you stop uploading videos? I need you.
Ok.. so you said that C and H share those electrons evenly... Okay... But why tho? I mean... Why is oxygen hogging the electrons whereas carbon is like: "Yeah, I'm good, we can share equally"?
RavenclawFTW lol I know right .. Sometimes that confuses me too but I guess carbon is less electronegative than Oxygen ... The more electronegative element essentially " hogs" the electrons ...am I right b
Look up the octet rule. Or Just look up electronegativity.
oxygen is more electronegative than hydrogen, so when O-H share electrons oxygen pulls- or "hogs"- the electron away from hydrogen. There isn't a big difference in electronegativity in a C-H bond so they share the electron equally :)
Carbon and Hydrogen do not share electrons equaly, the polarity is just not strong enough
The vidéo is full of wrong stuff
thus the more electronegative element has a slight negative charge and the less electronegative element a slight positive charge
Thank you so much for sharing all this material on youtube..you're a blessing. I love your videos and your examples are great, truely helps!
Quality set up and presentation. Much appricated.
this is great but your delta is the wrong way round
i didnt notice that until i saw this comment
sushi bear
what is *the delta?*
δ
Everything he said is wrong is actually not true. First, Hydrogen bonds can be intramolecular (within the same molecules) here are examples: Succinic acid, glycol, 2-hydroxybenzoic acid!!! Also, google "dihydrogen bond" you'll realize there can be H---H hydrogen bond. Also CH group can form C-H---O hydrogen bond.
Everything I said is wrong?!? Really? It's all lies? Of course not. You bring up some good points, but they're all extremely advanced points, with examples that often happen only very rarely. This video is a basic introduction to the topic. Most students don't understand the topic at all, so I'm trying to explain it in an easy way that makes sense at first. When most viewers struggle to understand just the basics of Hydrogen bonding, it would be a mistake to add in all the confusing exceptions that you mention here. Those are more advanced topics for later.
+Tyler DeWitt you could have mentioned that intramolecular H bonds can be formed in some rarer/more advanced cases instead of saying that H bonds MUST be intermolecular and any bonds formed intramolecularly MUST NOT be H bonds..because thats not true and can be misleading...
+Kelly C I could have, but I didn't. Because I'm trying to keep things simple and this topic is confusing enough as it is. If you're interested in teaching (and I hope you are!), the *most* important question a teacher can ask is, "What can I leave out to make this clearer?" Once a student understands the main point, you can always get more advanced. But if you throw in a bunch of advanced stuff and it gets confusing, that student will never have a chance to learn the more advanced stuff, because they're totally confused and turned off. If you're interested in my philosophy on this, you should check out my TED Talk on this subject.
+Kelly C The great architect Mies van der Rohe said, "Sometimes you have to lie in order to tell the truth." That's how I feel about science education. I have a PhD in this stuff, so I'm guessing that many things you think are true and certain, I've learned they're more complicated. If we want to communicate science clearly, we've got to decide--at each grade level--what is appropriate to leave out.
+Tyler DeWitt its actually the first time that i came across your videos. and by looking at the title and the description box, i didnt know the audience you are aiming at is the beginners so i was expecting some more advanced level of knowledge.
anyways, thank you for sharing a bit of your teaching philosophy! i will definitely check out your TED talk. it should help me with my final year project haha.
Amazing videos! Everything just seems so clear. Thank you very much!
Your explanations are clear and easy to follow. You're doing a good job. Keep up the good work.
Great, thorough explaination!
I love love love you! The visuals help so much, along with the fact that you are extremely clear!
Thanks for clarifying this. I was having a lot of trouble with this. You're great!
this is awesome! thank you for explaining clear and talking properly so its easy to listen
couldn't make this more understandable and easy to remember than you did in your videos. thank you.
you are great in teaching
Wow. I don't think I've ever understood a concept this well! Thank you for your videos!!
You're videos are very helpful... Keep them coming!!!
Thank you!!! I am so happy I have found your channel!
Don't ever change, Tyler. You are an educational hero just the way you are.
I love you! you really made my chemistry SO MUCH EASIER! Bravo!!! you're such a great teacher that chemistry illiterate like me could now appreciate the fundamentals of chemistry. you teach so smoothly and so clearly!!! more power to you!
love the format, love the explanation. keep'em coming.
That papers blending on the board is AMAZING! I know how it works but it looks like magic. Hahah I love how he discusses anyway. so helpful
Thank you so much, helped me a lot preparing for PCAT!
I have huge chemistry tommorow and this video saved me. Thanks and greetings from Polnad!
Good Explanation, cleared my long confusion!
Awesome job. You really cleared all this up, thank you.
Man, you are saving my chemistry grade. Thank you SO much for these videos.
You are phenomenal, I'm training to become a Chemistry teacher and your method of teaching is inspirational. Hydrogen bonding concepts never been this clear in my mind. Great job..keep up the good work
Your videos are extremely helpful, thank you!
Simple and precise! Thanks for correcting the common mistakes! The video was great!
Hiiiiii nicole
..........this is the first video I've found that actually explained this in a manner I easily understood. Awesome job, thank you!!!!!
Thanks for this! Really helpful and easy to understand
Amazing explanation. Answered every question I thought of while watching.
You're awesome I'm depending my whole chemistry on your videos haha! Please do a set of Organic Chemistry vids if possible