Hess's Law and Heats of Formation
Вставка
- Опубліковано 7 вер 2024
- How can we calculate the enthalpy change of a reaction without doing it? There are two easy ways! This is how we can make sure a reaction won't explode in our faces!
Watch the whole General Chemistry playlist: bit.ly/ProfDave...
Study for the AP Chemistry exam with me: bit.ly/ProfDav...
Organic Chemistry Tutorials: bit.ly/ProfDave...
Biochemistry Tutorials: bit.ly/ProfDave...
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDave...
Modern Physics Tutorials: bit.ly/ProfDave...
Mathematics Tutorials: bit.ly/ProfDave...
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT


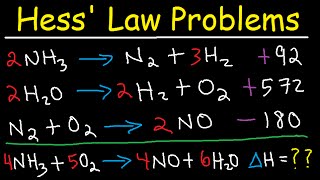






Sorry guys, there's an error! At 1:26, the second reaction with carbon dioxide as a reactant should have a change in enthalpy of positive 566 kJ. That changes the answer from what I've shown so please make a note of it. Luckily this error doesn't change the algorithm used, so apart from that you can still apply this concept to any set of reactions as shown here!
Professor Dave Explains I didn’t understand stand why we should change to positive it’s on the right side and no need to change
Thank you sir for the clarifications
Its ok professor
@@sahibahmed6732 it's because you must reverse the equation to get the 2CO on the product side of the equation, when you reverse the equating you flip the sign of delta H
@@jacoballan1153 I don't think that's the reason. I think he simply gave the wrong given for the delta H, that's why he said "Luckily this error doesn't change the algorithm used".
This channel is great! I get frustrated when I have to watch a 10-30 minute video just to explain a specific concept when you can just explain in it in less than 5! Really appreciate it
@Tyrone... that’s not lazy you need short breaks in between studying to retain information and w the introduction of technology attention spans have gotten shorter and it may be difficult for people to concentrate for long periods of time. They know what suits their studying stop judging people who are trying to learn. Conserving time and energy is not lazy. Wasting time on a long video is stupid when it can be condensed and less time consuming. Ur excused.
@mello why r u on this vie then lol
Dude you are actually right 👍🏻👍🏻
Ahh we have 1 -2 hrs video
@mello the human brain can only focus for 10-15 mins at a time, this being a complicated subject doesn't change that it CAN be explained in a simplified manner. They also just want to general rundown of the subject, not in depth details like 10-30min videos do. Too much information can make it difficult to understand the concept.
Who’s got an exam tomorrow >>>>>
14 years ago... Lol
😢
blud I got one in 30 mins
@@juniusq7963i have one tomorrow
😂
They just "taught" us this today and I was confused af and I'm so happy to have found a Professor Dave video on this. You never let us down man. From students worldwide, we love you!
You are single-handedly carrying my grade right now
did u get a good grade?
i love you so much man... I've been struggling to understand the Hess Law for months now and you are just a life saver!! You just got me subscribed to your channel
My high school chem teacher always called you hairy chest guy
you help me more than my professors sometimes thank you
I have been thinking of how amazing he explains complex topics and makes everyone understand for ages and now I got the answer. He speaks in a rhyme, uses emphasizes perfectly, and allocates us time to understand while speaking.
Thank you so much Dave for your videos they really helped a lot, I wrote my physics exam today and your intro song " He knows a lot about the science stuff, Professor Dave Explains" was in my head the entire time, lol and i think i nailed it, and now I'm studying for my chemistry exam 😁
You are an actual life saver, I am like 20% sure I have a quiz on this stuff tomorrow (it is currently 12:30am), and I didn’t understand it before now
This 4 min video is much better than the 1and a half hour lecture my professor gave me
My chemistry teacher stresses me so bad, she doesn't know how to explain her classes and many of my friends end up confused with the lessons. This video was short, simple and easy to understand thank u so much
should state what state they are in (l), (g), (aq) or (s).....for instance, NH3 are you talking about the (g) or (aq). Im assuming gas only because of the value you have chosen.
I also assumed H2O was liquid but they chose gas
Lots of thanks professor😊😊😊😊🇭🇺🇭🇺🇭🇺
When I say this I speak for the entirety of my senior AP chemistry class, you are a god damned national treasure
Dear Prof Dave, your podcasts including this one on Hess' Law are great. You have explained the chemical reactions so well. You are great and thanks for making Chemistry a lot more easier. Continue to do the great works with your podcasts.
How'd the rest of your chemistry class go?
you're my favorite teacher ever, helped me with maths last year and now with chem. youre a life savior prof!!!!
Great video! Super clear and precise with no wasted time. Much more efficient and easier to keep engaged than those videos that write everything out. Thanks
U have made this topic very easy in just about 5 min
Super work👍👍👍👍
I like it that the comprehension doesn't give any information and we have to find it ourself.
Thank you sir helped me revised... Would definetly mail for any doubts
Saved me for my exams... Thank you professor
Prof. Your video is very helpful even if it some is an unknown topic for me but when you explain it is understandable.
Yes, Dave, lets check comprehension.
At 2:20 the sum is also coming -1343. 0 kJ/mol
But the mistake defined by the Dave is neglected
Thank you professor dave you are a blessing to all students and learners 🙏
Prof Dave saves the day. My daughter was struggling with one of these problems and Dave had the answer. :)
We really appreciate your help
How do the O2 cancel put at 2:24, one equation has 4 the other has 2...? (i.e 2O2 and the other is just O2?)
yes im so confused
THANKS SO MUCH educational videos under 5 minutes are really just a life saver
still having hard time i understood the concept but when chemical reaction is given am blank please help
you must denote the state of the substance in the equation especially for water
cuz the standard enthalpy formation are differ if it is gas liquid or solid
I believe the equation displayed in the video is referring to water in its liquid state. For the gaseous state, I obtained an answer of 1170 kJ/mol.
My classmates call you chemistry Jesus
You teach everything perfectly in just 5 to 10 minutes....👍
Hi Professor Dave! Quick question: I find the H*f of water to be around -285 kJ/mol in every resource I see. Which source did you use to get -241.8 kJ/mol? Your videos have helped me study so much, thank you!
Haha I know this is 8 months late, but from my Pearson Chemistry Textbook it uses -241.8 kJ/mol for the formation of water as a gas, while it is -285 kJ/mol for the liquid. :)
so easily explained thank u
for the comprehension question, dont we need the states of matter?
You deserve to have more subscribers. Thank you for all the clear explanations. 💯✔
In the first example 2CO2 -> 2CO + O2 the Delta H si positive, not negative because the opposite reaction is exothermic. So the result of the calculation is incorrect.
Riccardo Magliocchi this is exactly what I thought! (and confirmed by Chem book)
One has to specify whether one is using the heat of formation for H2O; either (g) or (l), they are different.
He said standard temperature, which means around 20°C. Water is a liquid at that temperature so it was either a calculation error or wording error.
i don't understand how/why did you cancel the 2O2 and the O2 in the first example, I can't get my head around it
fast and clear. thankyou
I have a question! ---
So we know that forming bonds is exothermic (-deltaH) and breaking bonds is endothermic (+deltaH). If we are given the heat of formation for a specific molecule, but the molecule is broken in the reaction, should we reverse the sign (+/-) when calculating the overall delta H of the reaction or does it not matter? I hope this is clear!
I think you should use it just as it is given, without changing it. Hopefully someone else replies to confirm since I'm not that sure
thank you professor dave
You are very helpful and I watch you from egypt
Best and easy to understand!
Dave, thank you.
You explain everything so so well. Appreciate the help
Hey Professor Dave, Im unsure why we have to subtract Sum of the heats of formation of the reactants from the sum of heats of formation of the products to know how much enthalpy was needed for the reaction. When you break apart the reactants youre releasing energy that will be used (-) to form the products . heats of formation are positive for products and negative for reactantsb plus the negative sign of the subtraction you would be adding the heat released to the heat absorbed... shouldnt you be subtracting to know how much extra heat was needed?
Thanks professor Dave🎉❤
THANK YOU SIR YOU CLEAR MY DOUBT
I use your channel as a reviewer to recap what I've learned so far. And it helps a fuck ton!
For the question at the end, I used the standard heat of formation for liquid water, not gaseous water. D'oh! Also, didn't know. Also, thanks for all these great vids!
the heats of formation of the consistuentiants i found online didnt seem to be correct... although my mathematics seemed to be correct. well thats frustrating.
thanks a lot helped clear some confusion
thank you so much professor!! this helped me so much since tmr is my chem final exam,
Since H2O is liquid at STP, why did you use the number for it in a gas state?
Thank you sir ! Lots of love from India❤!!
thank you professor
Does heat a formation talk about the required energy to make that form out of regular disassociated ions?
Hess's Law? More like "Hella good lectures, for all!" 👍
I'm just a little confused when we use
Kj/mol
Kj
or when we talk about
Standard enthalpy of formation
Enthalpy change
enthalpy change of reaction
Is there a video talking about this more? and the differences if anyone knows
Is enthalpy of formation can also be zero for element if they are in same state as of their standard state even the temp. Is other than standard temp.(25°c)
(or STP condition)?
Gotta love the comprehension music
I didnt know science question then i watched video now i know. Good job👍
Why is the heat of combustion greater for larger alcohols but is the opposite case for alkanes?
Informative , and helpful.
Why is your value for water that of water in the gas phase here?
Can u explain standard enthalpy of formation and combustion. I'm a jee aspirant, please explain this it will be very useful
May I ask where did you get the -566.0 kJ from the given example?
For last part , why we have Heat of reactanct minus Heat of products ? .In hess law it is opposite version. Thansk
How do I know the value of the heat of formation of each compound?
Thank you chemistry jesus!!!
I don't understand, in the equations there is an unequal amount of oxygen, so why do we remove it still?
Thanks for the tutorial. In order to reach the formation enthalpy for 1 mol of products, what should I do? Thanks!
Hello from the other side !
I'm watching in 2020 , Thank you ❤
I saw somewhere that we cannot measure enthalpy of formation of methane directly but i can't tell why... can u plz explain that why we can't measure enthalpy of formation of methane directly?
you're literally a genius thank you so much
your video helped me so much+ i think that you are super cool person ! love from israel.
What's the difference between potential energy and enthalpy?
What is the difference between this and standard enthalpy change?
THANK YOU VERY MUCH
CHEMISTRY JESUS
I hope you know how much of a lifesaver these videos are 😭🫶🩷
Thank you Dave, very cool!
Thanks
when do i subtract and when do i add the equations?
You rock Dave!
Many thanks :)
Sir, Why heat of Formation of organic compounds are not measured directly?
Sweet Jesus thankyou
Sir you are great😊Now I understood Hess's law well..thnk u soo much sir🌝
bro u r so great at explaining hess's law! I wish I saw your video before I had to wing it on tutoring a student T_T
Thanks papa dave
thank you .
Hello, is there a way to calculate the heats of formation?
I have a science project where I need the heats of formation for diesel and biodiesel, and its heat of formation is not online.
The heat of formation is determined by using bomb calorimeter; diesel has an average chemical formula as C12H23 because it is a mixture of 3/4th of saturated hydrocarbons and 1/4th of aromatic hydrocarbons, hence there cannot be an exact heat of formation as the chemical composition differs. This site ( chembloggreen1.wordpress.com/2013/02/01/putting-some-numbers-on-the-diesel-engine/#:~:text=The%20heat%20of%20formation%20of,and%20%2D242%20kJ%2Fmole. ) has mentioned the heat of formation for diesel but there was no source. Biodiesel are complex organic mixtures from plants and animals often blended with diesel so the heats of formation can never be uniform.
What is enthalpy in simple word I don't understand enthalpy
Good
Very good teacher. You're saving my butt
You should have said the H2O was vapour and not water as I used the wrong enthalpy for it. No matter
THANK YOU
how do you get the kJ/mol???😢
professor dave just saved my ass before a test
Its so fun to watch your videos !😁