Hydrogen Bonding
Вставка
- Опубліковано 9 лют 2025
- Explanation of the feature required in a covalent molecule for the intermolecular force to be called a hydrogen bond. Followed by examples and a look at the three anomalous properties of water brought about as a result of hydrogen bonding








Answers for Chemistry Homework with Ms. Dana
1. What are the requirements for H-BONDING? 0:38
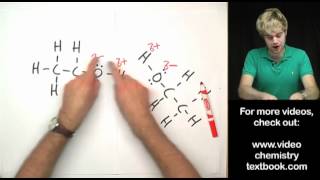
2. Draw hydrogen bonding in
A. H2O Water molecules 3:44
B. NH3 Ammonia 3:22
C. HF Hydrogen fluoride 1:55
3. Which intermolecular force is the strongest? 0:06
4. What properties are determined by hydrogen bonds for H2O? 4:16
5. Why is the boiling point for H2O greater than that for H2S? A labelled diagram is acceptable 5:19
Shokran
Hamza Al-Ali 3afwan
Wg457348 Have I helped you with your homework?
Wg457348 sank you
@@MaChemGuy my teacher assigned watching your video and answering these questions that I wrote as homework. I figured that adding the questions to links of when you mentioned them would help.
Thanks for the videos. After my poor performance last year, I have been looking for new ways to revise; your videos are proving to be excellent! Could you please update your F322 playlst?
You ever heard of a legend? Heard his name is MaChemGuy
Thank you so much for these videos
You're welcome, glad they help :)
Hi sir,
are you going to be uploading videos which detail the analysis section of f324 on NMR?
Raj Chatterjee By Easter hopefully
Hiya again,
So is the bonding on the chloroethane PD-PD because the molecule is polar?
thank you ahead!
+Harriet Nicholls Hi you! Correct but be very careful with your wording. The bonding in chloroethane (forces between the atoms) is covalent but the intermolecular forces (forces BETWEEN the molecules) are PD PD. Does that make sense? Can't have you dropping unnecessary marks can we?
ahhh okay i get it i get it! sorry i was confused
Thank you
Nothing on Metallic bonding or Giant covalent?
Best get that sorted!
I love you machem guy😭😂❤️❤️
i love you
TheBriansmith1991 Thanks for your very positive comment!