Chemistry Lesson: Molecular, Complete Ionic & Net Ionic Equations
Вставка
- Опубліковано 16 вер 2024
- Learn how to write molecular equations, complete ionic equations, and net ionic chemical equations. Soluble ionic compounds dissociate in to ions in solution, forming the complete ionic equation. Next, spectator ions are removed, leaving the net ionic equation.
WATCH FIRST:
○ Balancing Chemical Reactions - • Chemistry Lesson: Bala...
○ Double-Displacement Reactions -- • Chemistry Lesson: Doub...
WATCH NEXT:
○ Practice Problems on Molecular, Complete Ionic & Net Ionic Equations - • Chemistry Practice Pro...
○ Types of Chemical Reactions - • Chemistry Lesson: Type...
LEARN CHEMISTRY FAST & EASY:
○ Check out the GCH Mega Bundle - getchemistryhe...
COME SAY HI!
Website: www.GetChemistr...
Facebook: / getchemistryhelp
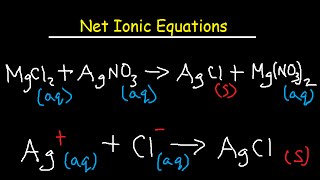
In this crash course, I cover how to write molecular equations, complete ionic equations, and net ionic equations. The molecular equation is a balanced chemical equation, often resulting from a double displacement reaction. The complete ionic equation, or total ionic equation, shows species as they are in solution by dissociating soluble ionic compounds in to individual ions. We use solubility rules to determine which products are soluble. Finally, spectator ions are removed, leaving the net ionic equation. For practice writing molecular, complete ionic, and net ionic equations, I cover several examples problems, including every step from predicting the products of the double displacement reaction, balancing the chemical equation, writing the complete ionic equation, removing the spectator ions, and then writing the final net ionic equation.







Great video! Thanks, and please keep up the great content!
If you learned then you like this mans video!
This saved me from the textbook, I am so grateful thank you!
Nice lesson! But I believe the chemical formula of hydronitric acid should be HN3 not NH3(ammonia).
No, the charge on the nitride ion is 3-, so it requires 3 H+ ions to counterbalance it resulting in a formula of H3N.
:D