Hess's Law - Chemistry Tutorial
Вставка
- Опубліковано 2 гру 2011
- This chemistry tutorial covers how to solve for the enthalpy of reaction for an given reaction by using Hess's Law and the delta H values for other known chemical reactions. This tutorial involves several examples demonstrating the use of Hess's Law, which allows for the calculation of an unknown enthalpy of reaction from other reactions due to the fact that enthalpy is a state function.
www.thechemsolution.com



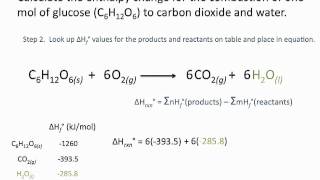





im so screwed for my quiz
Tries to teach you chemistry... steals your heart.
i love her voice. it makes chem really soothing to learn and less likely to give up :]
you saved my life. thank you so much. and u have such sweet voice.god bless you.
Perhaps the most seductive hess's law tutorial video I've ever seen
Honestly you make everything so simple to understand, probably the best video I've seen on Hess's law. Also I like how you showcase all your work. Thank you
The whole Arab nation is watching your video hahahaha you've helped us in chemistry a lot !!! THANK YOU SO MUCH
YOU ARE AMAZING!! what the Chemistry teacher failed to teach in 2 hours you taught it in less the 15 Minutes ,, Lady you deserve a Like And a Subscribe ,,Keep Up The BRILLIANT WORK!!
Please help us continue making FREE chemistry videos by clicking on the link below and voting for Big Head Business Solutions. It will take less than 10 seconds, I promise! Thank you! Thank you! ~Missy (The Chemistry Solution)
www.missionmainstreetgrants.com/business/detail/33553
your voice haunts me in my sleep
This was extremely helpful for me - I used to think Hess's Law questions were about making up the enthalpy numbers for the moles of reactant but then I saw the way you cancelled out the moles in the equations and it was like an epiphany! Thanks Missy :-) xxx
is this luna lovegood speaking?
I have a big test and this tutorial was AMAZING! I feel so much more confident now thanks!
Thank you!! Your method make so much more sense than the diagrams they draw at uni to try and explain it :L Really helpful!
Wow. 2 weeks of confusion cured in the first 3 minutes. Thank you. :)
what a clear perfect explanation. Thank you!!
Thank you! Learned a lot from your video than I did from reading the chemistry book.
So I have an exam on this tomorrow and the entire unit I had no idea what my teacher was talking about when he said to flip the equation and multiply and now it makes perfect sense! Awesome video thank you!!!
This was so incredibly useful! I understand sooooo much better now. Thank you! :)
OH MY GOD.
THIS VIDEO SAVE ME in my last 3 hours of the online submission DEADLINE
Thank you so much I have a chemistry test today and this stuff was so confusing, I even tried studying in the book and my notes but that got me nowhere, but this was an awesome explanation
THANK YOU THANK YOU THANK YOU!!!!!!!!! I was really struggling. This doesn't mean I'll pass my midterm tomorrow but at least I can do the practice problems without crying now!
Thank you so much for the tutorial! It was very helpful! :)
Thank you so much for making this video! It really helped with my homework & you have a calm slow voice its perfect!
thank you so much for the help. hopefully i wont fail my test. also i have to be the billionth person to say this but you have a fantastic voice.
OMG gurl, your voice is soooooo beautiful and flawless. Its just that your voice and the instructions make this PERFECT. =D
You should youtube a song btw, your voice is just... just... amazing
This made no sense in honors chemistry but I totally get it now! Thank you so much!! And I know the comments below already said you have a very nice voice but it is so soothing. Perfect video.
This was SUUUPER helpful. Thank you!!
WOW it's like I've crossed over to a new realm of understanding the HESS'SSSSSSSSSS LAW Thank You!!!!!!!!
Thanks, I was missing a few pointers and this video covered them!
Thanks so much! My chem teacher didn't explain it nearly as well as you!
you make this soo easy! thanks a bunch!
holy.... u saved me!! i never got this shitty law and now i got it
Really well explained, Thank you!
Fantastic tutorial! Thank you very much!
Excellent tutorial. Very precise and clear instructions.
Its guys like you the reason we dont get questions like these in the test a wrong answer, thank you so much
Thank you so much for this amazing video, very helpful, I feel that I'm ready for Chem exam
Thank you for this tutorial. It was really helpful :)
Thank you so much!! So helpful :) Finally understand this! 7 hours before the test lol
Thank you so much for the video! I get it now! It all makes so much sense!
thanks, honestly so much more simple than i was making it out to be
I don't know what it is but I started to fall in love with chemistry all of the sudden
THANK YOU. I actually understand this completely. Awesome tutorial. Way better then my teacher. :)
finally made Hess's law simple 😃😃😄😄
I totally master the Hess's Law now
Thank you! So much easier to understand in this video than the way my professor taught it
How come at 8:25 she didn't just multiply the equation by 10 to make the 1/2O2 into 5O2? I thought you just had to look for similar compounds.
Very effective and helpful!! Thank you so much!
You're chemistry tutorials r a big help! I have been able to comprehend everything i have learned in my chem college course, and yur tutorials make it a cinch to learn the material!
whoops i meant "your", not "you're"
Very Helpful, good job.
awesome vid, much simpler to understand here than in class
this video is a life saver ! thank you!
this tutorial was WELL done!!!!! Thanks!
First of all, I love your voice. Secondly, this was a very helpful tutorial. Thank you.
this helps a lot! thanks!!
Thank you so much. I needed this for an upcoming test i have tomorrow xD
The units of the reaction are not kilojoules per mole. It's just kilojoules because you have a coefficient of two in front of the reactants and product. If you divided the delta H by the coefficient then you would get kilojoules per mole
Any videos on specific heat problems?
is delta H measured in kj mol*-1
I dont get something from the second ex. the N2 is only once in the three equations too. can I strart modifying the quations with N2 instead of N2O5
Thanks so much! My teacher just confused me but this vid helped clear things up :) btw, I loooove your voice x)
Thanks girl, you explained it very well. ;) gotta know this for the SAT subject test.
For the first example, whydo you have to move CO2? Isn't it already a product for the initial equation and the first one?
wow thanks so much! so helpful!!
thanks a lot!!! great lesson!
beautiful voice by the way! come teach at my college!
Thumps up! i do like ur way of explaining!
omg u made everything simpler than my teacher
thank u so much
Thank you soo much that was needed :)
this helped so much thanks!
Super, super helpful!! Thank you!!
thank you vey much, very nycly explained
Amazing. Now I can understand.
Thankssssss, this video saved my life as I have an exam tomorrow :x
you clarify this very well! because the AP book does not state any of this extra multiplying so it throws off your answer thank you ;)
Thank you so much you helped me alot I have an exam tomorrow and I know everything now :-)
thank you so much. this was super helpful!
Excellent Tutorial!!
Sooo helpful! Thanks a bunch!
Good examples. A little harder than the straightforward examples I have seen.
You saved my life
Ur voice is my life 😍
Thank you so much. Much appreciated.
Loving the voice ... helpful vid . respect
Your voice is so pretty! thank you!
most helpful video thank you so much!!!!
Good job, keep it up!!!
Very helpful! Thank you!
love your voice
I also dont get why the last equation is flipped on the last exercise I NEED HELP!
Thank you so much! I was soooooo lost. :)
THANK YOU!!!! You have saved me :D :D
love all your videos! My chem teacher is from India and i can't understand a word of his lectures! I would be screwed if not for your videos(and others here on youtube!)
YOU'RE A GODDESS!
How did you cancel O(2) when it was 2:1 ratio.......?????????
You mean 4:58?
There are 2O2(g) on the reactant side and also 1O2(g) on the product side.
Therefore, we can cancel out 1O2(g) on both sides. Just simplify the equation. (please excuse for my poor english...)
thanks alot. i can understand it better now :)
I'm playing the video a 2nd time just to listen to her voice. I already know Hess's Law hahaha
OMG YOUR VOICE IS AMAZING!!!!!!!!!!!!!
Why did you change 2O2 (g) on the reactant side to 1O2(g) at 4:58? I'm confused.
There are 2O2(g) on the reactant side and also 1O2(g) on the product side.
Therefore, we can cancel out the 1O2(g) on both side. (please excuse for my poor english...)
hey why did you flip the last equation??