What Are Electrolytes?
Вставка
- Опубліковано 16 вер 2024
- People throw around the term "electrolyte" quite a bit, but what does it mean? What makes something a strong electrolyte, a weak electrolyte, or a nonelectrolyte? let's find out!
Watch the whole General Chemistry playlist: bit.ly/ProfDave...
Study for the AP Chemistry exam with me: bit.ly/ProfDav...
Organic Chemistry Tutorials: bit.ly/ProfDave...
Biochemistry Tutorials: bit.ly/ProfDave...
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDave...
Modern Physics Tutorials: bit.ly/ProfDave...
Mathematics Tutorials: bit.ly/ProfDave...
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT








Thanks Prof. Dave. I usually don't understand what my teacher says ( broken English and will scold you if you ask her anything) but your vids are simple so that allows me to understand. Thanks man.
Your videos inspire me to love general chemistry even more. Thanks Professor Dave.
Super helpful!! I'm taking a chem course in college and this helped me understand how the material I've learned is connected! Thanks
Elizabeth Dubinsky what college do u go to 😅😅 we learn this in 8th grade
DragoTPD : ThePyrusDragon in college you go over basics then go further and further until you don’t know what’s going on anymore and want to crawl in a corner and cry.
@@paarthjagga7287 its covered in a basic college chemistry course.
@@navjotsingh2251 facts
ty chemistry jesus
😂😂😂😂😂😂
" BRAWNDO got what plants crave! It got electrolytes!" ;) A heart if you understand!
It's what plants crave!
...the thirst mutilator!
somehow this comment makes my day
so i should drink hydrochloric acid if i need electrolytes?
haha no don't do it!
No, you have to mix it with water first
Yah!!! Go for it
You have to mix it with water first 😰
Dilution might help
I've been stuck on a Mastering Chemistry problem for a while now and this video helped me! :) Thank you!
Now I know how Charlie Brown felt when talking to his teacher
Wahh wah waa wahh wahh waaahh
Thank you very match.
Just I have a questions: what are the applications of electrolyte in the life?
And I would like that you mention the applications of any lesson you do
Thank you
Electrolytes are used in electricity production, particularly in galvanic cells and electrolytic cells. In these cells, the electrolyte allows for free movement of ions, thereby conducting electrical charge and allowing the cell to function
“Water? Like from the toilet?”
What for?
I’m a blood cancer patient, and I never heard of the word electrolytes until I got cancer
Are you okay now??
A 😊student from Pakistan. Excellent Sir.
You helped me a lot in my electrochemistry course.
Am I the only one who’s not getting it?😂
No
Not at all! I am very angry right now! :-)
I understood that electrolytes are nothing special, infact I’m worried electrolyte drinks will kill people sooner simple. Milk for example will have normal level of reactants. Instead people are drinking delayed acid aka a bottle full of electrically reactive chemicals.
I understand what he’s saying only because I’m not a novice with chemistry. This is very poorly explained to a novice lol.
I'm 8 th grade and I understand this
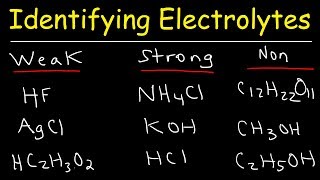
Dissociation and ionisation are different processes - you have used the terms interchangeably which would confuse students. eg silver chloride only dissolves/dissociates partially but is a strong electrolyte due to the fact that the part of the crystal that does dissolve is entirely dissolved as ions. Partial ionisation (eg as occurs with acetic acid) partially dissociating is a weak electrolyte since of the dissolved CH3COOH molecules most remain as molecules ad only few ionise into CH3COO- and H+ ions.
I don't think I used them interchangeably. Dissociation is for ionic solids, ionization is for covalent acids and bases.
@Professor Dave Explains 3:53
This kinda saved my chemistry test. (I’m studying to be a chemistry & physics teacher) and this was a bit fussy for me
I love your presentarion. Please share the electrical proses in human body. What electrolyte we need much.
Simple and fun. Loved it thank you genius
I can do not understand this video but your video instruction is great
"Honey, you are working so hard and sweating so much, you better drink a lot of electrolyte"
*hands over sulfuric acid*
Professor Dave I have got a doubt 🔊How HF is weak electrolyte.. Cause It has electro negative flourine and a proton like hydrogen...??
it's a weak acid, it'll deprotonate
@@ProfessorDaveExplains 👌🆗 tqsm
How nicely you taught sir i am now Cristal clear about this topic.... Thanks a lot... Actually i m from India....
Dave you are like my go to on anything I don't understand in school ily and your videos
Used this video to study for my exam thanks for the help
4) Name the electrolyte is used in a salt bridge and write the condition required for its selection.
thank you sir i am having thes on 23 of October relay helped thanks
Anyone who is a fan of the movie Idiocracy has heard of the word "electrolytes" before.
" Brawndo's got what plants crave. It's got electrolytes."
Thanking you so much Dr.dave ,it was a wonderful talk ,I m bit curious to know how did you put effects in this videos, like background is running and all kindly enlighten me so, I can also benefit society
with regard
suhaan
According to my sources, all acids and bases are electrolytes so are they all covalent compounds and dissolvable in water? If they don't dissolve in water, does it mean that their ions don't disassociate either; are some acids/bases that are not electrolytes because of their inability to disassociate in water?
It might be a dumb question but how to know the molecule’s electricity? How to know if it’s a weak or strong or non just by looking to the names?
like if it's a strong or weak electrolyte? well usually strong vs. weak is just ionic vs. covalent, but there are plenty of exceptions, like insoluble salts, or strong acids, so really you just kind of have to know them.
@@ProfessorDaveExplains please make another video that is more complex+more detailed
Hi sir, This is the first time ,i saw ur video...the intro song is super, it attracts me
Science is cool when you keep it simple......Hope this helps!
How do you get fluids out of the body (legs) due to edema?
This is great!
No one gonna talk about MGR
thanks sir
great work
This leacture is very very helpful in pendimic situation sir jee 👍
Is water electrolyte ?
I LOVE YOU
YOU ALWAYS HELP ME THANK YOU PROFESSOR DAVE
URAL FEDERAL UNIVERSITY
Surely, I can’t be the only person with a huge crush on Professor Dave…🤭
great !!
Thanks brother ❤
I want see a prectical example . Thanks your helping me out from....
It's September 1st 2021 currently UA-cam movies has Idiocracy for free.
I just watched it with my 14-year-old boy. Good times
i understand the concept but I still can't categorize them o.o
Proff Dave>>>
السلام عليكم انا احتاج الى مصادر موثوقة وفيها معلومات كثيرة عن المحاليل الالكتروليتية أنا احتاجهم في بحث للجامعة ارجوا المساعدة
thanks sor ji ☺☺☺☺☺☺
Sir copper coin is dipped in electrolyte solution of sulphuric acid and now the copper coin ions charges are activated
What about solid electrolytes? I am confused, since an electrolyte was defined as a liquid solution.
Thank you!
Thank you jemsus🛐🛐
He had me at "sports drinks"
I love this video. Thanks a lot sir
What electrolyte is used for tubular batteries
Are all minerals that we consume considered to be electrolytes?
Mast video ha sir thanku
What about foot edema. Professor dave does it keep the swelling down
😳
Thank you, sir!
Hi ...did acetone strong electrolyt ??? Thanks
Thanks
Nice exp
Why HCl is a strong E, & HF is a weak one?, they both produce H3O, so why Prof Dave?
HF is a weak acid
@@ProfessorDaveExplains maybe a video about calculating the pKa would clarify why some acids are stronger than others. Do like your channel a lot! Thnx
Yep check my general chemistry playlist I have other videos on acid base concepts
Thanks 😊
Phosphate, sodium, calcium, magnesium, chloride.
As always...You're The Best°°
at 4:28 you say "donate a proton" is this correct because if so I'm confused cause I thought elements couldn't lose or gain protons outside of nuclear stuff?
I understand now. I didn't put it together on my own that an H+ ion is literally just a proton since hydrogen doesn't have any neutrons.
Ps. I'm leaving my dumb mistake in case anyone else has the same thought.
is electrolyte potiental mean how much electric current pass through the electrolyte?
Please Make videos on differential equations too.
Super👌👌Dave
Sir can you please tell me when I Dissolve NaCl in water then NaOH and HCL is Produced ?
no, hydroxide is a base
@@ProfessorDaveExplains but sir we know that when ever NaCl is Reacted with water then Na ion and Cl ion is separated by water molecule
So Na + H2O =NaOH +H
And with the remaining H is reacted with Cl so HCL
Can you please explain it sir my teacher can't that's why I asked you
hydrochloric acid is a strong acid, it dissociates in water, so chloride ions won't pick up a proton in aqueous solution
@@ProfessorDaveExplains thanks for your explanation
Thanks teacher I am Ethiopian citizens
Why HF weak electrolyte although it is the product of the intreaction of an element in the first group with an element in a seventh group or that the hydrogen with bromine an anomaly??
HF is a weak acid because of how small the fluoride ion is.
Is vinegar an electrolyte
I was expecting hydrogen fluoride to be a strong electrolyte in aqueous solution like hydrogen chloride. 3/4.
HF is a weak acid because of the relative instability of the fluoride ion compared with the other halide ions, due to its small size.
@@ProfessorDaveExplains Thanks for the reply. Yes, I just watched your "Acids and Bases, pH and pOH" lesson and it is crystal clear for me now. I am studying about chemical corrosion of rocks and minerals and the term electrolyte came when I was looking at one of the processes of chemical corrosion which is hydrolysis. All of that to take the term of electrolyte. Thanks for the lesson it is very helpful.
Superb
Is gatoraid good for electrolites?
Is a summarization for the process and steps for it?
*is there
los of a electron or gain of a electron
Electrolytes, its what plants crave!
Protons are transferred and not electrons??? I thought when protons are transferred it would be nuclear reaction how come protons were transferred from the HCl?? I was just asking because im really confused
watch my tutorial on acids and bases, a proton is what we call an H+ ion.
I can't hear you. Your intro music though is fine though
ur intro is more cringy than bear grills doing yoga
Bro you lost me
can water ruin electrolyte?
is there a correlation between Acidic solutions and strong electrolytes?
No, both acids and bases can be strong electrolytes.
@@ProfessorDaveExplains Ok thank you
I will pass my exams
Are acids too electrolytes?
Does it mutilate thirst?
After watching this I’m confused. Need a easy to understand simple definition
love your intro :)"!!!!!!
You rock dude. Thank you. ☺
got to get the electrons to flow from atom to atom on the far outer circle
Very thankful for your pronouncing 🙄 keep up good job man very good
Electrolytes conducting electricity is permanent?
i couldn't even edge to this! i exploded immediately, clean up on aisle my pants! 🍦🍦🍦🍦🍦🍦🍦🍦🍦🍦
Thanks professor