How to Calculate the Molar Volume (Vm) of a Gas
Вставка
- Опубліковано 4 жов 2024
- I'm inviting you to join AttaPoll. Get paid to take surveys. Download the app here: attapoll.app/j...
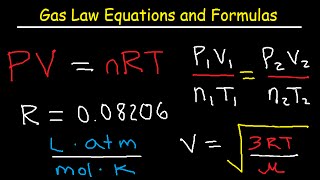
The molar volume of a gas is the volume occupied by one mole of the gas at a specific temperature and pressure. The ideal gas law is often used to calculate the molar volume of a gas. The ideal gas law is given by:
�
�
=
�
�
�
PV=nRT
Where:
�
P is the pressure of the gas (in Pascals or atmospheres),
�
V is the volume of the gas (in liters),
�
n is the number of moles of the gas,
�
R is the ideal gas constant (
8.314
�
/
(
�
�
�
⋅
�
)
8.314 J/(mol⋅K)),
�
T is the temperature of the gas (in Kelvin).
To calculate the molar volume (
�
�
V
m
), you rearrange the ideal gas law to solve for volume:
�
�
=
�
�
V
m
=
n
V
Here's a step-by-step guide:
Convert the temperature to Kelvin if it's given in Celsius. The conversion is
�
(
�
)
=
�
(
°
�
)
+
273.15
T(K)=T(°C)+273.15.
Substitute the values of
�
P,
�
V,
�
n,
�
R, and
�
T into the ideal gas law.
Solve for
�
V.
Divide the volume (
�
V) by the number of moles (
�
n) to get the molar volume (
�
�
V
m
).
Remember to use consistent units throughout your calculations. If the pressure is given in atm, make sure that the gas constant (
�
R) is in the appropriate units (L·atm/(mol·K)).
Keep in mind that the ideal gas law assumes that the gas behaves ideally, which might not be the case at very high pressures or low temperatures. At such conditions, deviations from ideal behavior might occur, and corrections may be necessary.