Shed Synthesis of Lithium Niobate
Вставка
- Опубліковано 22 тра 2024
- A microwave synthesis of a photonics computing material from the obscure element niobium. Subreddit: / explosionsandfire
Join the Discord!! / discord
Patreon: / explosionsandfire
Music: All tracks by the artist Corticyte !
/ corticyte
Track 1-
Track 2-
Track 3-
Track 4-
References: - Наука та технологія








The music in this episode is by an artist called Corticyte, check them out here: soundcloud.com/corticyte
do your thesis
friendship ended with aphex twin
now corticyte is my best friend
@@trinityghoul1660 He said he was gunna do it, so he will. There's no need to remind him every 6 months...
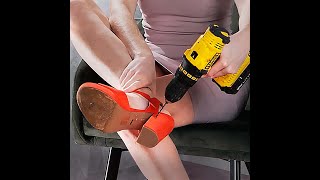
You need a welder's reference for adjusting an oxy-acetylene welding torch so it is neither carburizing nor oxidizing (same principle will apply to that rosebud torch or a cutting torch)
@@TheDuckofDoom. yes I need a lot more practise with the oxy-acetylene flame!
NileRed, Nurdrage and Extractions&Ire in the same day. The chemistry gods smile upon us.
Now we need Cody's Lab video.
We need a chemadelic finishing his synthesis of... Well we don't mention what precursor he recently made can be turned into.
ThatChemist also posted a tierlist, but chemistry related nonetheless
@@stephensteele3553naughty chemistry
@@jackingwads7513 naughty chemistry is best chemistry
For a hot minute I genuinely thought a "Shed Synthesis" was some advanced chemistry technique I'd never heard of, maybe discovered by Dr Gustav Shed.
It was first pioneered by Florida Man and has been kept alive by countless disciples as well as a diverse group of YT content creators .
Long live their eyebrows !
glad I wasn't alone in thinking that
kkkkkk Dr. GUSTAV SHED kkkkkk
Wasn't him the guy who invented chemical peeling? XD
Shed Synthesis aka shake and bake
hmm yes Karl Gustav Crack-Shed, I can almost see him working in his cottage in Bavaria.
Well, there's only one thing for me to do I guess. Make a protein based OPO, but make it so it's only emission is bright yellow.
Exceptionally petty revenge plot, I love it
tar black/brown is a good alternative
That's some funny shit 😂
He’d love that.
Do it
Tom, re-melt the lithium niobate you made but use an oxygen rich oxidizing flame instead of the reducing fuel rich flame you used, it will oxidize out all the carbon that it got impregnated with and clear up. Don't be afraid to get the whole crucible glowing hot, it just means a slower cooling time that will yield better crystals.
I was going to say the torch looked a little oxygen starved but your comment is better. I agree.
I don't know anything but this seems right
@Daniel Cook 😂🤣 he'll do better next time!
Should also add, don't light an oxy-acetylene torch with another flame, use a proper cup lighter
@@NeneExists why is that?
"600-900 degrees. It's not super hot". Spoken like a true solid state chemist
My oven broils food at 550 degrees, so I'd say these temperatures only really classify as "quite hot" as opposed to "super hot", it's only slightly above to rather above cooking temperatures...
@@MaverickBlue42your oven probably broils at 550°F, which is nowhere near 600°C
@@MaverickBlue42 600C is over 1100F. Much hotter than oven temps.
@@frostlips22 I laughed. I was imagining them turning food to charcoal.
@@oafkad hey if you want your oven to double as an iron smelter I'm not judging. You can have your sword and eat it.
Here's a fun fact about niobium, it's one of the most problematic trace contaminants in steel for the future fusion reactors. Nb has a huge neutron capture cross-section and it gets activated into a nasty Nb-94 isotope with 20000 year half-life and high decay energy. As a result, normal steel activated by fast neutrons from fusion will have to be treated as a low-level nuclear waste basically forever. One of my classmates worked on a project to make low-niobium steels, but they kinda gave up on that.
At least you get your money’s worth with a 20,000 year half life.
Now...is it the engineered niobium alloy that's the problem? Or the natural contamination?
@@nunyabisnass1141 Just the natural contamination. It's enough to make the steel too radioactive to be re-used in non-nuclear applications.
Dooooooooope thanks for the info 😊
Funfact about HF-: it’s a scavenging reaction in which it hydrates the positive cation of whatever and then proceeds to recycle the hydrogen back into the fluorine and leaving the cation to self bind and reach equilibrium away from the reaction.
When I was a kid I wrote to a Niobium mine not too far from where I lived to see if they would send me some for my elements collection.
They didn't send me pure niobium, but they did sent me a cool shiny rock of ferroniobium.
If it's not in Brazil, I most likely work in that mine. Is in in québec by chance?
@@jean-francoisgagnon8379 It is indeed!
@@hugoboyce9648 That's funny finding a comment mentioning my workplace. It's very satisfying to see videos about niobium knowing it may very well come from the mine I work at. There is only two other mines in the world and both are in brazil.
@Jean-François Gagnon so can you hook our boy Hugo up with a specimen or what?
@@rehanb637 Unfortunately there is only ferroniobium made there since it's used in alloys.
NZ based organic chemist here - I share your sadness about the loss of plastic utensils from supermarkets. I use a lot of sodium azide and needed some plastic spoons because the azide forms explosive compounds when exposed to transition metals. Ended up finding a lot of plastic spoons at a chinese takeaways and pocketed them :)
I use wooden spoons for that, which I rub a bit of wax on to (stops absorbing of any dissolved azide). Not the greatest solution, to be sure, but better than metal!
The reason why it went black is because your flame wasn't neutral. It was orange. You need to give it a little more oxygen until the flame turns blue then it won't deposit Carbon on anyting
Yea I cringed when the flame was splitting.
yep. he needs to torch it again with an oxidizing flame to burn out all the carbon and recrystallize his product.
Can still be reducing and blue. Can also be oxidising and blue. Neutral is adjust the oxygen to make the white cone as small as possible.
You know it's a good episode if he needs 2+ new equipment for it 🥰
You know it's a forced comment when they use 🥰 unironically
@@oshkiv4684 luv u ❣️💗
Let's see how long until he accidentally "offers 20V for a 12V expensive equipment, because it could grab what it needed to work..."
@@ruffusgoodman4137 setting both regulators to max because the acetylene flame will just “grab what it needs”
@@oshkiv4684what
Lithium-Niobate was the second harmonic-generating material I used for frequency doubling a 844 nm laser to 422 nm for my senior thesis in college :) nice to see it getting some love
Only thesis? I make a living growing these crystals :)
Can't say czochraski process is quite as straightforward as taking a blowtorch to it (unfortunately).
I’m sorry but harmonics are absolute aids and your thesis sounds like torture
Is it like a tweeter medium? Does it decrease pulse length as wellwell?
Stargate fan canon:
O'Neill: Carter! Tell me in ENGLISH how that defeats the Goa'uld?
Carter: I am making a visible laser pointer for my presentation, sir.
At my old job i was in charge of the nickel niobate synthesis, for that synthesis the homogeniation of the powder was extremely important. We ball milled the Carbonate and niobium pentoxide together for ~16 hrs to homogenize it. We also used extremely large kilns, that hit 1600C, but idk if the that temp would be transferable to getting purer lithium niobate
Shoutout to the people doing research on these esoteric compounds that have like 6 useful applications. Incredible work! 100% +/- 10% yield! Thats way better than single digits
Its used in piercing jewelry and Tiger tanks from WW2 and this. I wonder what the other 3 are
@@off6848 I’m sure someone will discover another use for niobium after they throw a dart at their periodic table to decide what to research.
There are a few gem cutters who facet pieces of Lithium Niobate into gemstones. They are quite beautiful since they have a refractive index and dispersion much greater than diamond, so they sparkle with insanely bright colors. The only catch is their Mohs hardness is only about 5.5-ish, so you wouldn't want to have them set in like a ring or other heavy-use jewelry.
You could set it inside a magnifying vial with an LED light source inside.
the cat slapping around your metals was by far the best part of my day.
Actually, you can get hot enough with your microwave, I played with this about 20 years ago (for fun) and I reached about 2400°c . I used a old analog pyrometer from the sixties so not ultra-precise but I have definitely melted alumina.
I used polishing grade coarse SiC as the microwave absorber (susceptor), I think it is the black thing rather than graphite that is placed inside your kiln but there is not enough to absorb all microwaves (it is on purpose i guess).
My advice in the same kiln put a thick bed of alumina power put some SiC powder on top ,cover it with alumina powder and then put your alumina crucible on top (of course try to have a good contact area with crucible and the powder below but without direct contact crucible-SiC).
If you put the alumina crucible directly on top of the SiC, it will shatter due to the thermal shock.
Damn. That's some serious microwaving.
Also, since theres standing waves inside microwave it is good to place kiln to anti-node, where most of the power is concentrated
Probably would work in an older microwave. These newer microwaves are weak sauce.
@@johndeerekid167 The one I used was 800watts
I played with it and got paid for the priviledge too! Used a similarly ancient kitchen microwave to make lithium battery cathode materials and even got it to print in 2000! Went through alot of devitrified quartz and kept our glassblower busy ;-)
Niobium makes for really great nickel superalloys as well. There's a ton of interesting research being conducted on them in the aeronautics industry
I did not expect to see you there 😂
@@Pxx500 Oh hey lmao. Likewise
@@elisis isn’t the whole discord server here?
Tom's Shed Microwave: What is my purpose?
Tom: You heat up dangerous chemicals until you die
Microwave: *oh my god*
i love the term "shed synthesis". that's such a good word for what you do.
There is nothing considered "weird" that is beyond this man's challenge!
Next week: DIY Fluoroantimonic Acid!
Don't tell him that Fluorine is yellow.
@@frednurk5168 i don't think he's crazy enough to mess with fluorine
probably a sore point, but what about Cubane? That's been a challenge...
@@matteocdt5214 I see what you did there!😆
Tip for the acetylene torch. Your inner flame cone is too large. Less acetylene or more oxygen to get a neutral to oxidizing flame. Right now it's more carburizing. You're putting carbon into your product. Also for lighting it: half a turn oxygen then quarter turn acetylene to start with, adjust it from there. If your pressure is to high or low the flame will go out. To turn it off, first the acetylene then the oxygen. If you do it the other way around you get a lot of soot that is actually a little carcinogenic. And for the love of fuck don't light it with a liquid or gas fuel lighter. Use a striker. There's a tiny explosion every time you light your torch. If this damages your lighter it can catch fire in your hand. Which can be kinda bad since the torch is blowing oxygen at it.
If you smother you torch by accident and the flame goes into it. Just turn it off like normal. The torch will start getting hot pretty quickly as the brass sucks the heat out of the flame and it travels up the torch. If it goes past that into the hose, shut off the valves to the bottles. to prevent them from further turning into little rockets.
be safe and happy lighting shit on fire!
The way Niobium hardens steel , is by making the grains in steel very tiny which makes steel harder but not brittle! It's kinda crazy
Why so little of it does the trick, though?
@@ruffusgoodman4137 I guess it takes a single or a few Niobium atoms to help start nucleation, so you don't actually need a lot, or maybe magic.
@@your-mom-irl almost certainly magic
Is this considered grain boundary strengthening?
@@ruffusgoodman4137 kinda reminds me of just how little lead they needed in early gasoline to prevent knocking
Really excited to see this video from my favorite chemistry youtuber! I just had my PhD thesis on using lithium niobate piezoelectric resonators for power conversion.
After just finishing a class on superconducting RF cavities where Niobium seemed like the miracle element that makes the whole field possible its very funny to hear it being considered not very important
It was very important. The entire fiber-optic telecommunications network was built on it. I believe it was phased out. but Lucent churned out a ton of Lithium Niobate laser modulators - they couldn't build'em fast enough. I THINK the amplifiers also used an LiNO3 substrate, but I can't remember anymore.
Is that the horseshoe quantum tunnelling device that could be used for qubits or smth like that?
"Lithium Niobate is an optical material. It was used in the 60s I think? to make the first OPO"
Fuck me I love the 60s
That was a nice video!
I've actually worked with amorphous niobium oxide (sometimes called "niobic acid"), which I believe is the exact material you made here (didn't make it myself, we ordered it from a supplier). It has a very high surface area, and can hold on to a non negligible amount of water, even as dissociatively chemisorbed species (i.e; surface hydroxyls). We used it as a support for catalysts.
So that extra water might explain why you got 100% yield despite the mechanical losses.
And cool to see you got an acetylene torch! You may want to try making an oxidizing flame, so that you don't leave carbon on your metal oxides... (I think "cutting tips" are designed specifically for that. I've only used those torches to seal glass ampules though, so don't take my word on it.)
that is not what a cutting head does. Has neutral flame then pull lever and get pure oxygen in center. the material you have heated up begins to burn in high oxygen environment. the metal itself burns rapidly.
@@humphreybumblecuck5151 Well, in that case, pull that lever and the pure oxygen would burn off any soot or tar before it can even deposit on the material being melted.
There was also a little crust on the niobium metal left over, which could be heavier than just the niobium content.
@@piranha031091 problem is it's too effective, the temperature is so high most things will burn/degrade 3500 Celsius.
Just use a normal head, there are 2 knobs one is oxygen one is fuel. Start with a neutral flame and then just open the oxygen a little more to get an oxygen rich flame which does have it's uses.
This method will also oxidize things but has the benefit of being slightly lower temperature and more importantly not a pure oxygen environment.
Go look up what an "Oxygen Lance" video and be impressed with what a little heat and pure oxygen can do.
Or the Apollo 1 fire
Niobium is a great addition to certain knife blade steels as well. It helps form a finer grain structure. A little bit goes a long way, too. S110V has about 3%, for example.
This man gives the vibe of an innocent side character of a show who ends up being master manipulator behind everything
Jeepers man, you really are everywhere
@@mikedrop4421 He is the one who comments
@asgrahim9164 well, I mean
What do you do all day heisenberg? Comment on 1400 channel uploads?? Why are you everywhere??
@@asgrahim9164 a guy opens his door and attempts to use sulphuric acid to dehydrate some chicken nuggets and you think that of me? no! i am the one who uses a freeze dryer!
YES! I LOVE VIDEOS ON ELEMENTS WHICH I'VE ONLY SEEN ON THE PERIODIC TABLE
An Extractions & Ire release and a Nile Red release within the same hour. The stars have aligned.
I'm a big fan of lithium niobate crystals. If you put a c-axis cut under weak vacuum (like 10^-4 torr) and cycle the temperature the uncompensated charges exposed on the ends act like a particle accellerator and you can achieve electron and ion beams up to ~200KeV. That's without any moving parts or high voltage. If the crystal is radially symmetric the beam self focuses. If the residual gas in the weak vaccuum is deuterium and you have a deuterated plastic target you can even make a linear accellerator fusion device for making small neutron fluxes. Definitely a fan.
Tom: "People in the comments will have done their entire PHD thesis on Niobium"
Me in the comments who has no PHD at all and only comes here for Tom's personality and for him to call things cunt: "Yea... I totally know what Niobium is"
Camera work in this one really nice, the sequence of setting up the torch for the first time was more professional than anything I expected on this channel and it honestly caught me off guard. Great video mate, perfect mix of beauty and jank
Haha thanks!
For a cleaner product after melting with the torch you will need to have complete combustion of the acetylene. This is a achieved by using more oxygen as I’m sure you know. If you watch a UA-cam video about acetylene welding I think it would help you get the flame profile you are looking for
Very amusing that the cat saw identical metals that are hard to visually distinguish and immediately stirred them with a paw, truely masters of malice.
True niobium lover here, I am doing a PhD on niobium alloys
First Nile, now Tom uploads. This smells like a conspiracy. A ... chemistry conspiracy!
Also Nurd Rage uploaded today too.
Now we only need Cody's Lab video.
@@Mirrori I thought nurdrage uploaded yesterday.
If you had a neutral flame on the torch it would've melted better, it looks like you were running acetylene rich by how orange the flame was. For a neutral flame you want to balance them so that there is a single bright cone in the middle of the flame, and most of the flame should be blue with just the end of the flame starting to turn to orange
Niobium isn't obscure! It's a part of one of my favourite minerals - columbite-tantalite (or coltan), which is (Fe, Mn)Ta2O6-(Fe, Mn)Nb2O6. This mineral is primarily mined for the tantalum therein, as it is used in capacitors in modern electronic devices, but niobium has a lot of extremely high-tech, science-y applications too, mainly in the steel industry (where it is alloyed with stainless steel to give it strength at low temperatures, which is handy for a lot of aerospace applications*), superconducting magnets (600 tonnes of Nb3Sn alloy is in the Large Hadron Collider, while the International Thermonuclear Experimental Reactor uses 600 tonnes of Nb3Sn alloy, and another 250 tonnes of NbTi alloy), and high-performance lithium batteries. It can even be used to make people's glasses thinner, as it increases the refractive index of glass.
*The Gemini and Apollo space programs both relied on niobium to make their rocket engines, and SpaceX is currently using such an alloy for the engine of the upper stage of the Falcon 9.
I've got very thinned glasses due to my natural magnifying vision. So I could have been watching this through niobium salts! Cool.
They also make T-800s out of coltan 🤣
A big use for lithium niobate is as the substrate for making bulk acoustic wave resonators, which are signal filters used in cell phones and other digital communications hardware.
Niobium alloys are common in rocket engines because of the high temperature material properties. They're a bit of a pain but sometimes you just need it.
I’ve never felt so secure about watching someone else try to do the really challenging and frankly dangerous and impossible things I can only dream about. Never ever stop being You Sir. You’re an inspiration 😊
Oh yeah he pushes himself with his own lab equipment, that's what I like..
I like niobium. I have a few big chunks of it. It's a neat metal, it can be oxidized to form some nicely colored layers on its surface (due to interference in various thicknesses of oxide). It's also biologically inert and used for jewelry, piercings, surgical hardware etc. But that's not why I have it. It's also a grain refiner and very strong carbide forming element in steel. Sorta like vanadium. I want to use some of the niobium I have to make wootz steel, i.e. ancient Damascus steel.
The world needs more science like this maybe shedology 101, keep up the awesome work man.
Niobium was named after Niobe, the daughter of Tantalus, the Greek character the element Tantalum is named after because it’s so similar in properties to tantalum
How could a Greek god possibly be similar to it?
@@Ewr42 wdym? Tantalus’ daughter in Greek mythology is Niobe. Since Niobium and Tantalum have very similar chemical properties, they named the newly discovered one as the “daughter” of the already-discovered tantalum
@@spiderdude2099 bad line break I guess, i really struggled with "Tantalus, the Greek character the element Tantalum is named after because it's so similar in properties to Tantalum"
@@spiderdude2099 Lots of others are named this way too.
Titanium (Titan) Uranium (Uranus) Plutonium (Pluto)
As a gem cutter and jeweler, niobium is awesome and lithium niobate is amazing as a cut gem!
I enjoy this so much! Amazing content, best wishes to you and your future projects! 🌻
I remember seeing that niobium has pretty looking oxide, a sort of violet or indigo. At least, that's what I recall it being from reading about it on Wikipedia ages ago
NbO2 is a beautiful navy blue color. I thought he might have had some of that in it at first, but it looks like it was just a complexing effect in the acid or something.
I care a lot! Lithium Niobate is one of my favorite gem materials.
A Nile vid AND an Extractions and Ire vid on the same day?!
We have been blessed
The Way He Said I Don’t Wanna Go Into Made Me Feel Like He Really Wanted to Go Off On a Tangent to Talk About it in Depth…. 🤦♂️🤷♂️😂😂😂 Live These Videos & This UA-cam Channel!!!!!
What a perfect resolution to a shitty day in the lab than unwinding to a video of Tom doing transition element chemistry
May look into HHO torches to reach your fusion temperature. Nighthawkinlight had one that was desktop-sized and could fuse alumina into rubies.
21:27 You are a comedic genious. The way you placed your glove over the heat exhaust of the microwave here had me cracking up! :D
i took an art class at my local college and they had these microwave crucibles, they put ceramic shards on the plate to allow the crucible to continue to rotate without damaging the plate. also i noticed in that class that different microwaves heat faster so if you hate the 30 minute wait you should consider testing your friends microwaves and buy the hottest one you tested.
i would like to thank u for ur decision to start off this pride month with some Nb representation on ur channel.
🤣
With everyday the PhD grows closer to completion. Keep going!!
In college I was an intern on the Stanford Free Electron Laser facility (now torn down) which used a niobium superconducting array as its electron accelerator.
youre amazing dude. a year ago, you single-handedly re-sparked my interest in chemistry. youre a natural comedian, combined with being an all around smart human being. yours is one of my favorite channels on youtube. i hope you keep creating some form of content, whether its funny chemistry videos or phds in chemistry or physics. just brilliant! (im american btw, we dont use brilliant to describe cool stuff and its reserved for only the brightest people).
It's always an adventure watching your videos wondering how long it will take you to break down into explaining the process with "We're gonna do something with some amount of stuff for some period of time and hopefully that works 🤪" We got there in 6 minutes this time, that might be a new record, I have high hopes for the rest of this video 😄
ive heard a lot that you generally shouldnt use a flame to light an oxy-axxetylene torch because if the gas flow blows out your flame youre now filling the area with toxic flammable gas mix. also going to echo the others in the ocmments saying your flame was a reducing flame because you didnt have enough oxygen in it, and the excess carbon from that got deposited on your product
So I just got done watching a NileRed video, and the way each of you go about things is so vastly different, its both jarring and amusing
you're one of my like, favorite people I've never met. Thanks for making videos, you're rad as hecc.
Attempts inorganic chemistry.
Generates tar.
Always a great day when there’s a new Tom video!
If you want to get real heat out of your acetylene torch you need to adjust the gas ratio until you have short (a few millimeters), sharp blue flames. Then holding it within a few centimeters / a centimeter of what you are heating. I learned to cut with a torch and adjusting the flames to be short and intensely bright increases the heat (and cutting in speed in my application, not so applicable to you) significantly. Using enough oxygen is key to complete combustion and will cut down on the soot output too. Heating the crucible could get the job done too, but the correct gas mixture should eliminate most of the soot issue.
Really good to see you posting. It’s been a while. More please and more explosions
i actually cracked a liebig once using a forced air condenser when distilling sulfuric acid (similarly to how you did a few years ago, right on the jacket), id recommend using a vigreux column instead and keeping the sulfuric at such a rate so that the convection cooled vigreux can keep up with it. that glass stir bar had me worried a little since glass can scratch glass and with hot sulfuric acid you dont really want score marks on the glassware for obvious reasons, though i still probably will buy some as theyll be perfect for something i have planned. as for that SO2 like you mentioned its probably the reaction, ive dissolved copper in hot sulfuric acid and the sulfuric acid gets reduced to sulfur dioxide while the copper oxidized to copper oxide which then forms copper sulfate so that blue tint may have been niobium sulfate or sulfite but unfortunately i cant find pictures of those so idk. cracking the flask like that ive done too many times, its not a good idea to heat a wet flask with a flame or run liquid onto hot glass like that, as for melting maybe an arc furnace though that may spit graphite in from the electrodes, neat video like always
I refine all my sulphuric acid from old car batteries and I use a 400mm air condenser and put a 200mm liebig on the end of that with water cooling and it's never been a problem.. u use broken crockery for boiling chips but you need stuff with a course structure to minimise bumping and that's never damaged my boiling boiling flasks.. a little tip if you're distilling old battery acid is if it starts bumping to much I've found stopping and letting it cool to about 100c before filtering through a Fritz helps a lot, sometimes it needs to be done twice to fix the bumping.. I have no idea why the filtering helps but it seems to work
>100% yield
>starts turning yellow
hmmm
Awesome to see some new Extractions&Ire! super cool use of a microwave as a rudimentary kiln, i wonder hot hot you could go with some additional modifications, perhaps focusing the magnetron's waveguide to the centre of your crucible in some way~
In solid state synthesis it's usually a good idea to get your particles as close together as you can before sintering/firing. Our lab commonly uses a hydraulic press to press the powders into pallets before they're heated. Since you are going above the melting point of lithium carbonate, and the reaction is thus not entirely solid state, paletting might be a bit of an overkill.Still getting it ground as finely as possible and than compacted should help. Also it's completely normal that the grinding/firing stage might have to be repeated several times, some solid state synthesis require up to a dozen repetitons of this steps to get decent yields.
I think a lot of papers repeat too many times just to look like they're serious business though
Some people will probably do that. But I don't think it's really super common. The grinding and refiring costs time, money and sanity. So in a decently equipped solid state synthesis lab, you would do a powder XRD after each firing-grinding, and stop the process as soon as you get a pure (enough) phase.
Hey! I work in natural user interface, robotics, and computer vision. A while back when you were talking about your phd you were talking about OPO and it really cracked my head open on silicon photonics, which has changed my life. I completely forgot what you called the spinning prism by the time I got to the other side of that rabbit hole but a couple days ago I saw someone making a little portable laser galvo setup out of an old hard drive and just started geeking out about spinning a prism or a series of prisms and mirrors on the dead hard drive that was sitting on my desk but I could not remember the thing to google. Anyway, thanks for before and thanks again now. You just keep hooking me up. You're a saint and I'll drop you some stacks on that Patreon for it. Tell my girl to put you on the holiday card list. Lol.
Caught it right as you went public with it. This is gonna be a cool one. It also makes an excellent gemstone, although IIRC it's a bit soft for a ring. Good for earrings and pendants.
After watching the video, I think you may need to go ahead and melt all your product but heat it longer, leave it in the molten state for a longer period with the torch, then dissolve it and re-crystallize the product to grow actual crystals of the product for clarity.
Honestly, nothing makes me happier than these videos.
Thank you
Something else interesting and exciting about photonic computing is that recently a group over at Stanford was able to implement back-propagation, which lets you train neural networks. For like, AI. And shit.
Yeah lots of interesting things coming along!
So happy to get more extractions and ire/fire vids while you are procrastinating your thesis. 😂 For real though best of luck/skill on your doctorate sir!
Thanks! Still being very slow on it but I am making progress!
Wait wait wait, your phd is in physics, and you do exclusively chemistry content. Styropyros degree is in chemistry, and he does almost exclusively physics content. Wtf.
Glad someone was there to guide ya on that kit. Acetylene is some dangerous shit if you don't know what you're doing. Glad to see another vid!
Try putting a lid on it and blasting the outside of it with the torch. It would be better to have some enclosure around the whole thing to retain the heat as well. Might be able to prop one side of that microwave kiln up and place the flame to swirl around the crucible .
I use insulating firebrick. You can carve them out with a spoon , although hole saws make quick work of it. I use 3. One for the base, one with a hole through it and a hole in the side at the bottom for a propane torch tip, and one brick for a lid with a smaller hole for the exhaust. I’ve melted aluminum many times this way, and for you, it would allow a semi controlled cooling by simply turning the flame down a bit over time.
Moon is going to explone on monday.
Don't be schizo
Ok guys can we just chill out
Nerd
nooo
We use plenty of niobium... mainly in the NbTi alloy for superconductive magnets, cables and resonant cavity coatings. Sheets of the alloy would also be good for magnetic shielding, but softer materials like lead or aluminum are much easier to handle
E&I is like a box of Whizzo Quality Assortment, you never know what you're going to get next. It could be Crunchy Frog, or it could be the Steel spring surprise.
I used to be a niobium man myself. Built RF cavities for linear particle accelerators out of it. Definitely in my top 3 superconductors.
I watch every channel and am hyped for each installment.
styropyro:gets chemistry degree, but makes laser
Extractions&ire: gets degree related to making lasers, but does chemistry
I really love the mad scientist working in a shed with trash equipment (no offense) vibe we get here. This channel deserves more views. It would probably help to "spice" up the production value (but keep the vibe!).
Binge watching your videos back to back- and adding comments for the algorithm~! 🌻👍 Keep up the great content!
Well Tom, I took the entire transcript of this video and copy-pasted it into GPT3.5, and this is what it had to say:
"Thank you for sharing this detailed episode on the extraction and use of niobium. It seems like you had an adventurous journey in working with niobium metal and converting it into niobium pentoxide. The process of dissolving niobium metal in sulfuric acid, followed by the formation of niobium pentoxide and its subsequent reactions with lithium carbonate, demonstrated your exploration of different chemical reactions.
The use of a microwave furnace to achieve the required temperatures for the reactions, as well as your attempt to fuse the niobium pentoxide using an oxy-acetylene torch, showcased your resourcefulness and experimental approach. While the results may not have been as expected, it's clear that you enjoyed the process and learned along the way.
If you have any specific questions or need further information about niobium or any other topic, feel free to ask!"
Shake the Future has an excellent UA-cam video on smelting metal in a microwave, including how to make a crucible and how long it takes to melt various amounts of metals. In particular, I think it's worth trying the microwave furnace without the brick in the oven absorbing a ton of energy and slowing down the melting of the metal.
Bingo! I knew it was was the Niobium, that goldish luster in the light is a dead giveaway. Some idiots might confuse it with the Tungsten but not this laddy. Fantastic video, as usual.
With a NileRed upload, and most definetly this upload, I've scratched my chemical curiosity itch. No words to say thank you for the amazing content!
Might have been already suggested but in terms of melting the lithium niobate you might wanna look into getting an HHO generator. The flame size is generally much smaller than oxyacetylen but it can reach similar temperatures without the introduction of any carbon. I have used it to melt glass on several occasions without the need to pre-heat the glass. Got it off of Amazon for like 200€.
The reason I like your videos is because you bumble along without proper research and try stuff just like I do but you have such a different field to me it feels like you're a bona fide alchemist!
Huh, what a happy coincidence, I just recently got some faceted iron-doped Lithium Niobate. Apparently used in research as a holographic storage medium. Sure is a pretty shiny rock, too. Would definitely like to hear more about your PhD thesis when you're able to.
Niobium fan here! I don't know much about the element itself but I have a 1cm cube of it on my desk because it's anodized purple and I like that color :3
I had a chuckle at the bit with the acetylene torch, we have one at my workplace and sometimes someone using it will forget which dial is for which gas while working and you'll just hear the bang
I used to work at a chem plant that dealt with Niobium! Glad to see someone loves it
There were some strange materials used back then for lasers; one was trivalent uranium doped calcium fluoride, which Peter Sorokin developed while he was at IBM. It had to be cooled with liquid helium, and it lased in the infrared around 2.5 microns when pumped with a flashlamp. It was the second laser developed after Maiman's ruby laser.
new to this channel, first vid and already loving the content. i hope the crusty fan makes more appearances