Thermochemistry Equations & Formulas - Lecture Review & Practice Problems
Вставка
- Опубліковано 16 лип 2016
- This chemistry video lecture tutorial focuses on thermochemistry. It provides a list of formulas and equations that you need to know as well as the appropriate units. It provides a nice review covering topics such as the internal energy of a system, the surroundings, endothermic vs exothermic processes, work, pressure, and volume. It explains the difference between work done on the system vs work done by the system. It also shows you how to calculate q, the amount of heat absorbed or released by a system for processes that involve a temperature change or a phase change including the specific heat capacity concept of water. This video also discusses thermochemical equations and reactions and how to do thermochemical stoichiometry and conversions. This video contains plenty of examples and practice problems.
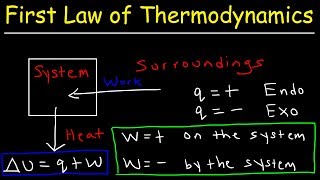
First Law of Thermodynamics:
• First Law of Thermodyn...
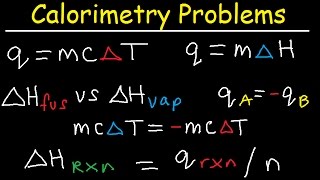
Thermochemistry Equations:
• Thermochemistry Equati...
Internal Energy, Heat, and Work:
• Internal Energy, Heat,...
Thermochemical Equations:
• Thermochemical Equations
Specific Vs Molar Heat Capacity:
• What Is The Difference...
________________________________
Basic Calorimetry Problems:
• How To Solve Basic Cal...
Final Temperature Calorimetry Problems:
• Final Temperature Calo...
Latent Heat of Fusion & Vaporization:
• Latent Heat of Fusion ...
Coffee Cup Calorimeter:
• Coffee Cup Calorimeter...
More Calorimeter Problems:
• Bomb Calorimeter vs Co...
__________________________________
Specific Heat Capacity Problems:
• Specific Heat Capacity...
Hess Law Problems:
• Hess's Law Problems & ...
More Hess Law Problems:
• Hess Law Chemistry Pro...
Enthalpy of Formation & Heat Combustion:
• Enthalpy of Formation ...
Enthalpy Practice Problems:
• Enthalpy Change of Rea...
__________________________________
Speed of Light, Frequency, & Wavelength:
• Speed of Light, Freque...
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections







Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Videos & Worksheets: www.patreon.com/MathScienceTutor/collections
My thermochemistry test is next period and this is my first time finding out what thermochem is.
Uh, how'd it go?
@@incognito6751 lol good question
@@incognito6751 Not too good I assume
Me too and my test is at the end of class 😁
DUDE SAME
I just wanted to let you know that you are an outstanding teacher! If everyone had you as their prof, no one would complain about chemistry being too hard! Thanks so much for making these tutorials public.
U have to read😮❤ newton,s precipia
@@michaelgonzalez9058That would probably be a life-long endeavor. 😅
You got me through calc and are getting me through chem. You're a celebrity in my eyes
That helped me understand a lecture of 2 hours in 21 minutes, thank you so much, Online Classes just aren't for me
We had an 8 hour class for this, I just skipped it and watched his vids
Same
Bro is this not an online class
True, in my opinion, teachers and professors take that long on purpose like they could have finished earlier, but they decide to finish nearly at the exact time because of institutional guidelines that they have to follow including the pay per hour rule.
Same
I've actually been teaching myself all about thermochemistry equations and your videos are so clearly spoken that it simplifies it all. Thank you for sharing your gift.
if youre watching videos of it youre technically not teaching it to yourself. LOL
My lecturer took 3 weeks to cover this and confused everyone. You did it 21 minutes and i understand perfectly
thermochemistry test tomorrow, wish me luck my friends ( still not prepared lmao )
How did it go, fellow chem major.
Random Single Guardsman He dropped the course :/
she*
Best of luck dude
Are u Korean?
I have learned more from him than any chem professor
I feel the same, but you gotta remember that repetition is key to remembrance. Maybe your professed layed the groundwork by exposing it to you and this vid help build upon it? Or maybe they're just bad professors haha
yes
I can’t count how many times I’ve had trouble in chemistry and this guy saved me.
Just an FYI for you, towards the end of your video when you have the equation (-393) + 2(-286) - (-785) ..... You calculated that 2*-286 was 576.. but it is actually 572. which would make the actual answer to the equation be 180. just thought i would mention.... LOVED the video though, really helped me better understand.. I have a midterm on Saturday .. here to hoping I pass.
Thank you so much for this, it hopefully will help me pass my CHEM 1800 mid term the course moves way to fast so summarizing important stuff into a 20 minute video such as this is a great help!
Add some more videos on thermochemical calculations but tough ones please please...
I'm a 8th grader trying to learn thermochemistry and you're helping me more than you could ever know
Thank you so much
This this was a better explanation than my book,, thank you so much!
if u read this comment you're getting a 5 in AP Chem
sweet
by you saying that it's obvious you got a 2
amen
our grading system is reverse and 1.0 is the highest grade. 5 meaning you're probably on academic probation lmao
Engineering**
My thermochemistry test for ib chem is in 25 minutes. This man is a blessing
uni gen chem finals tomorrow im right there with u
You inspire not to use a calculator every time. I love this. This helped me a lot. Thank you so much
NOir Blanche i
can't use a calc on the A.P test sooo....might as well (multiple choice section at least)
Chemistry is sooo much info, first you need to learn all the vocab & understand whats going on, then memorize the equations to finally be able to do the practice problems 💀 but atleast if i dont learn from my prof, I can learn from other teachers on youtube like this one.
I hate ths bro i wanna die
Honestly I'm so thankful for your videos. Definitely helps soo much!
18:25 2×-286 = -572 and so the answer is -180. And great thx for video!
Ikr! I was solving with him, so i thought i got it wrong. Noticed his mistake too. But the video helped me so much!
Thank God i'm not the only one
Thanks
I almost got a heart-attack!
yup noticed this too 👍🏾
if I just simply watch these videos, my basics are going to get SET FOR LIFE, I didn't know it was this simple 🎉
Somebody has to pay you more for helping out so many freshmen :D
Keep making videos man, you have saved my chem grade
Awesome man! This is a great review of thermo helped me alot!
Thank you so much! God bless you more sir! You are a big help to us students!
Great Video! Saving me in my General Chemistry class in college
Thank you so much for posting these videos free!
i have a quiz on this tmrrw and was struggling so much, but you helped me and have helped me all semester, thank you
Thank you so much, way better than my prof!
Thanks JG. You helped me so much with a doubt about the sign - of PV. Salutes.
Loving soooo much your way to explain!
I've never for once comment on UA-cam videos, but for this, u deserve a medal 👏👏
Thanks alot
thanks a lot for the best explanation....clear and simple to understand
Thanks for the video, it really cleared up some confusion around the formula of ∆H. i'm still puzzeling with sensible Heat and Latent Heat. But this was a great start. Thank you
The numerator denominator thing is a huge help since I cannot for the life of me do unitary method without feeling uncomfortable.
Also ty for the amazing vid.
Don't usually comment so take it for every one of your vids I watch.
I love what you’re doing great stuff man
Am so much happy finding out much on thermo chemistry, and for the second to the last equation, the answer is 180kj/mol
Thanks for all your videos is real helping us I'm from Nigerian Turkish Nile university
Thank you so much for all the videos
I love your videos! Good to always be learning to work smarter in life! God bless the multiverse!
Hey brother just wanna say your teachings are amazing. God bless you mhen
if there were hundreds of subscribe buttons I would definitely subscribe them too. I cant tell how much these videos help. Your videos are gold. THANKYOU
I appreciate that you take the time to solve the equations without using a calculator. My chemistry professor isn’t that cruel, but my calculus professor IS. All these little tricks to combining very large numbers without a calculator has been a lifesaver! You and Professor Leonard have gotten me through my last semester as an undergrad.
All of your video helps me on my engineering course
I am a shs student infact because of good tutoring I stopped spending huge amount of money on vacation classes infact you helped the poor peoples who can't pay for classes😢 may God bless you
Thanks a lot man! This really helped me bro. Like it makes sense now. Bless!
you should do the sample with the units maybe for students wondering how you went from g of water and C degrrees to J.
Studying for MCAT and this guy has been the goat throughout high school and uni
You teach better than the text. Soon, teachers and professors of chemistry will surely enough begin jumping onboard to your channel for quality lecture content, only for the teacher/professor to work out a few example practice questions on the board for the remainder of class
you can be an amazing professor i know i would pay attention to in your class i love you thankyou for saving my grades
My hero , this will help me with my finals tomorrow so much
Oh gosh this was soo helpful! Thank you so much!
You make chemistry easy. God bless you.
I wished I watched this before I took my thermochem exam! Studying for the ap chem exam now.
You are fantastic!! Thank you for this!
sooo helpful! thanks so much, God bless you sir
Thermodynamics quiz tomorrow, thanks.
you're the best, man
God bless you fr... got my thermo exam tomorrow at UT.
Have a great day as well! Thank you
Paving my way to AIIMS delhi. Immensely motivated
Thank you for these videos
I love you so much
You are fantastic
God bless you man☆
For any topic and course that i learn something from you, i send a huge amount of positive energy
To u ☆☆☆☆☆☆☆☆☆
I HAVE EXAMS TOMORROW IM GOING TO CRY
haha
Me too,😭😭😭😭😭😭
Perfect as usual 👏🏻👍🏻
Thanks bud, really helped!
Thank you brother. Great video. 🤝
Highly appreciated,thanks
I feel like your voice helps me understand this, idk i love it though THANK YOU for your help
thought that it was my head playing tricks with me but jah, his voice does have a certain quality about it.
amazing!!! thank you
thank you organic chemistry tutor.
I'm love chemistry so much
can't thank you enough man!
thanks for this video fam
Goodluck on your exams!!!!
I have a question, why can I not just use q=m x delta H (54 g of ice times delta H?) Because you mentioned that we can do so when there is phase change?
absolute gold
I very much enjoy the way this man says the number 40
God bless you you actual lifesaver!
very helpful!!!
Just amazing
How do u relate current,time and molecules in thermochemistry
Thx it's extremely useful (TBH than school chem lol)
I appreeciate the mental math lessons incorporated in this video lol
hope my school recruits a teacher like you
2 * 286 should be 572 not 576 note it, but we found your video so helpful keep it up.
thank you!
Great video!!!
😜
Thank. You. Sooo. Much.
You are a amazing teacher ❤️ 💕 ♥️ 💖 💙 💗 ❤️ 💕 ♥️ 💖 💙 💗 ❤️ 💕 ♥️
My boy is doing Gods work
you are my hero sir
you are amazing thank you sm
Thank you!
Thank you
What's the difference between the deltaH definitions where one says 'products-reactants' and the other 'bonds broken - bonds formed'. It seems backwards to me. And when I used your deltaH definition for the complete combustion of butanol, I got an endothermic reaction! What am I missing?
Thank you king
Just wanted to let you know. When doing total standard enthalpy change for a reaction, the final units are kJ not kJ/mol since you are multiplying by moles.
This is really important because units are a tell tale sign of whether you’re doing the calculations right or not.
on last problem the Hf for 2H2O should be 572, not 576 :)
why would someone dislike these videos?? seriously ...