Intermolecular forces - ion, dipole, London dispersion and hydrogen bonds explained
Вставка
- Опубліковано 1 лип 2024
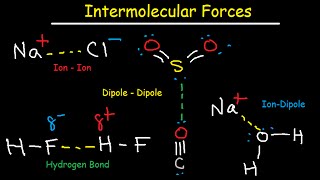
- Intermolecular forces are crucial to understanding solubility and miscibility, so let's make sure we've got the fundamentals right before we progress. In turn, solubility will help us all to understand varnish formation and additive precipitation, while miscibility will help us understand flushing and compatibility.
- Наука та технологія








Looking for more structured lubrication courses? Join LE Pro for $30AUD per month (that's about 20USD). lubrication.expert/product/le-pro/
You are a very gifted teacher and I wish I had had many more teachers like you throughout my life. While I am intelligent in my own way, I often have a difficult time with long-term retention of information and with the ability to relate past-learned information to newly learned information - and that is where your consistent repetition and reinforcement of information and lessons really helps me. I wish businesses and organizations of all types broke things down and explained them as well as you do and I think they do a great disservice to themselves by not doing so. I wish you great success in this passionate endeavor of yours and hope you get some great sponsorship in the future.
Thanks as always for the great content!
Thanks for the feedback! I'm working on trying to reduce those connecting phrases - but if I can't do it then eventually I might have to cut them out in the edit with software or something.
On sponsorships - I'm hoping that I'll never do that on this channel. It feels like if I can keep everything "brand agnostic" then it gives the lessons more validity?
Yay. Mr. LE's, On to it :) This Thesis, could explain, Preferential action, Upon working, metal surfaces?...
Between, Detergent & ZDDP, molecules? All Good.
That product/R^2... Coincidentally? Like Newton's Physics?...
Instantaneous means? Faster than The Speed of Light? Across, Known Universe?
How would that work? Ooo... Who could Figure That? :)
Yes! So the interaction between metal surfaces and additives is something I'll need to cover. But because the electric field within a conductor is always zero, electrons sit on the surface of conductive metals like steel and copper - this negative charge on most surfaces is what allows polar additives to be attracted to them.
Hi, Joe! What about the mechanical shearing polymerization of VIIs? Do they become a worthless sludge at the end of a Long Term OCI?
A little bit. Not sure if you saw it but we covered VI improvers in a previous video: ua-cam.com/video/-sL1aQw8xZk/v-deo.html