pH pKa RELATIONSHIP | PHARMACEUTICAL CHEMISTRY | GPAT | NIPER | PHARMACIST EXAM
Вставка
- Опубліковано 5 жов 2024
- 📢GDC CLASSES APP available both for Android📱 and iPhone📱users👩🏻💼🧑🏻💼
📲 GDC CLASSES APP for ANDROID📱
Download from Below link
bit.ly/youtube...
Free Batch Codes for GDC Classes App :-
Weekly Test - gdcquiz
Pharmacist - pharmacist
Drug Inspector - gdcdi
Pharma Helping Hands - help21
____________________________________________
📲 GDC CLASSES APP for iPhone📱
Download from Below link
apps.apple.com...
🏢Org Code for iPhone = GDCCLASS
_____________________________________________
B.PHARMA SEMESTER MCQs (PCI SYLLABUS) @ 1/-
GDC DAILY PHARMA QUIZ @1/-
bit.ly/youtube...








Mr. Your explanation is excellent because you did not use math, but explained the concepts and possible answers. My big thanks.
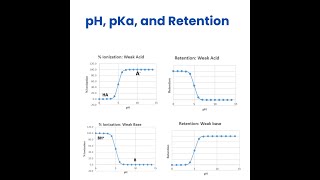
pH increased means to where.... Either from 7 to 1 or from 7 to 12?
Please clear it
pH range is 1-14. pH increases means towards basic side (towards 14 side)
How you calculate ionized and unionized ??.....You not explain their ......Please clear it 👍
By using henderson hesselback equation
i am not from Pharmacy. know some chemistry.
1. what do mean by medium's pH? that means its based on water?
2. you say pKa of drug. That means its not based on water. But in such case you referring to basic and acidic drugs, that means does pKa values indicate any material either acidic or basic? i mean what values of pKa refer to basic or acidic? [similar to pH > 7 is basic and < 7 is acidic]
pH of the medium can be neutral, acidic or basic.
How they determine the %ionised and %unionised value with (pH-pka) ?
Plz explain 🙏
that should come from the original equation as he mentioned initially. i.e. pH = pKa + log ([Ionised] / [Unionised]).
For basic drugs why you considered the ph 3,4,5,6?
very well explained.
thanks
If it will be little practical can effective more.. Still good
Thank you for your continuous support.
HAPPY LEARNING
superb sir. thank you so much
Sir, Ye pata hai, kalcutation kaise karna wo batao .
Thank you sir
Need screen shot of board
I think that percentages are just for approx... Or calculated??
Thank you so much
Thank you very much sir!
Phosphorus lowest pka value
Thank you sir