sp hybridization | AP Chemistry | Khan Academy
Вставка
- Опубліковано 7 вер 2024
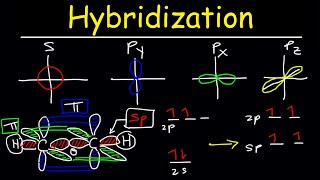
- In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. View more lessons or practice this subject at www.khanacadem...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacadem...
Volunteer here: www.khanacadem...








Didn’t know I’m going to need it after 6 years but yeah here I am 🙌.
So thankful being able to get such well explained videos👍🏼
Thank you for the videos! I'm studying for my matriculation exams (Like finals for Finnish students) and I was very confused about sp, sp^2 and sp^3 hybridization.
The word "THANK YOU" is just not enough ..!! You are great sir .. hats off !!
Do you have any videos on "Hybridization of elements involving d - orbitals" ?
It might be there in there chemical bonding playlist
Steric number is the number of atoms bonded to a central atom of a molecule plus the number of lone pairs attached to the central atom, not the number of sigma bonds. Acetylene has 3 sigma bonds and you only counted 2 which got me confused. I'm assuming in acetylene's case the 2 carbon atoms are considered as the "central atom"?
You are counting the number of sigma bonds around an atom eg, the carbon on the left. The steric number does not belong to the molecule, but instead belongs to a specific atom in the molecule - the carbon. There are 2 sigma bonds and no electron pairs on the individual carbón so it has a steric number of 2
I can't thank you enough if I had a credit card I would have donated
God send 🙏🏻 thank you Sir
Khan explains it much better
So does this dude!
Maybe you don’t understand because you haven’t seen his other videos? I think he explains better than Khan. The narrator of this video does not give examples in which you get lost... He gets straight to the point
I disagree. Khan does a fine job, but this guy is more concise.
Never compare teachers .
(Except ones in school they suck)
@@effortlessschool you had me in the first half
Explained well but should have mentioned that a triple bond consists of 2 pi-bonds and one sigma bond.
The primary cause of the shortening of the carbon-carbon bond is because of the pi bonds, not necessarily the increased s character -- even though that is a part of it, and is the reason that the carbon-hydrogen bonds will be shorter as well. If the primary cause was the increased s character, the carbon-oxygen bonds in CO2 would have near equivalent bond length as the triple bond, but they do not. I just wanted to mention that in the comments bc I was slightly thrown off when I heard that. Great video!
You have explained it so well sir
SN = Number of atoms surrounding the central atom + Number of lp of electrons that's why Acetylene has (SN = 2 +0 =2)
Allah razı olsun
Turkcesi yetmedi ingilizcesiyle mi anladin
I really can't say anything you are more than a great teacher love you😍😍
I don't get how you get two SP hybridised orbitals. When you combine 2 orbitals that should make 1 new combined orbital. How do you get 2 ?
free science lessons also quite good too
thank you so much, God bless you
i wish you were my proffesor
6:22 can u draw the H atoms on an unuybridised p orbitals?
dont care about hate comment! you r so much better than my teacher lol sorry not sorry
Thank you!!
Do u have any videos for of drawing hybrid orbital diagrams?
thank you
Hey Jay, I have a question about the formation of 2 equal sp-hybrid orbitals that are opposite in orientation. How is this even possible since the formation of an sp-hybrid consists of an s-orbital of singular phase and a p-orbital of 2 opposing phases. Wouldn't one side of the sp-hybrids have a much larger electron density than the other side as that is the side that constructively interferes with the s-orbital? So how then do 2 sp-hybrids form that have equal and opposite electron densities? Thanks.
I struggled with this too, but i then realized that the demotion of a p orbital carried its single electron and the s orbital also remained with its single electron. So, in reality you have 2 sp orbitals with a single electron occupying both. The linear orientation comes from the repulsion stemming from the single electrons occupying the remaining 2p orbitals and the pie bonds they create to prevent rotation. I could be wrong, but thats my reasoning.
Can anyone tell me why the pi bonds prevent rotation?
Hey there! The pi bonds prevent rotation due to the electron overlap above and below the plane of the atom! The molecule wouldn't be able to rotate freely around that axis due the two overlaps. I personally think about it as giving the atom more "structure". Hope that helped!
@@Bailliemariee Finally... I understood.. Thanks!
thanks sir
these videos are just amazing! thanks a lot!!
Thank you very much
Thank you 😍😍🙏
so single bond is sp3, double bond is sp2 and triple bond is sp?
Single bonds are sp3, sp2 are 2 single and 1 double, sp is 1 single and a triple or two double.
@@Arpier you just cleared out this shit so fucking easy
Thank you.
Why are we talking about the free rotation??
I know this is academic but half the sources I consult say that rotation about triple bonds is possible and half say it isn't. Any ideas on what the actual truth is?
They CANNOT rotate..
Pi bonds cannot rotate without first being broken.
No. In Pi bond no rotation.
Rotation is possible for sigma bonds. Pi bonds cannot be rotated as they will have to be broken first since they are formed only in addition to sigma bonds.
WHY there's only 1 electron in 2s orbital? Shouldn't there be 2 electrons in that orbital?
Its excited and promoted to the higher 2p level
i still dont get it omg
Arabic translation please 😭❤️
you can use the auto-translate subtitles feature to translate the subtitles into any language that google supports.
czech subtitules dont work :(
Can you explain it actually instead of this slow plodding bs taking 10 minutes to explain something that takes 1 minute? christ. Its too slow to keep anyone engaged
play it in x10 speed 😊
khan does better
talked to fast
make speed x0.5