Why You Can't Copper Plate Steel ~ Reactivity Series Explained (works the same for aluminum plating)
Вставка
- Опубліковано 22 сер 2024
- I'll show you the science behind acid copper plating steel and the extra steps required to achieve success. Watch entire video for all the info!!! All this information applies the same way to aluminum.
In chemistry there are several factors that go into whether a chemical reaction will take place. A couple pretty big hitters are the relative electronegativities and the position of the elements in the reactivity series. The way these characteristics interact with each other isn't necessarily intuitive especially if you don't have a very strong background in chemistry.
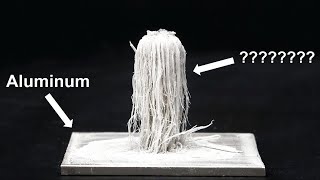
Basically since iron (Fe) is higher in the reactivity series and more "electro positive" than copper it undergoes a single displacement reaction with copper when it is placed in an aqueous solution of copper ions.
The reaction is almost instant and a thin coating of copper is formed on the surface of the iron. This prevents any kind of electroplating one might be trying to accomplish.
The solution to prevent this reaction from occurring is to first plate the iron component in something else before attempting to electroplate with an acid copper solution.
I explain in this video how the reactivity series works, how the immersion deposit of copper through a single displacement reaction occurs and what material to first plate onto the iron to achieve an adherent coat of copper plating.
Nickel plating is or tin plating is usually the intermediate coating process used to electroplate steel. I'll be making a nickel plating guide in the future so make sure you subscribe so you don't miss any of the info.
Basically nickel does not react with copper nor does nickel sulphate react with iron. This stepping stone process allows anyone to get good copper plating finish on steel parts.
#garagescience #diyprofessional #garageplating #diy #doityourself #chemistry #experiment #science #garage #scientist
If you enjoyed this video let me know in the comments or leave me a question if something wasn't clear. Below are some resources for your own reference. Good look and happy electroforming!!!
Reactivity Series:
www.thoughtco....
en.wikipedia.o...
Single Displacement Reaction:
en.wikipedia.o...
Electroplating / Electroforming Guide (Not for the faint of heart!)
metalfinishing...
Get Copper Sulphate and try this experiment yourself here:
amzn.to/2RzjEFT
Be sure to check me out on Facebook here:
/ garagescience
And on instagram here:
@garage_science








This is Karl, Karl watches a UA-cam video without providing feedback in the comments. Karl doesn't do UA-cam right. Don't be like Karl!
You CAN copper plate steel, but you need basic plating solution instead of acidic. Copper is soluble in basic environment only in complex ions, to my knowledge. Cyanide baths are most popular in the industry, but there are many other safer and easier ways to achieve good adhesion of copper layer to steel base. For example you can make a bath consisting of 20g copper sulphate, 200g sodium hydroxide, 50g glycerol, 100g tri-sodium phosphate, 2g benzotriazole. Current density up to 2A/dm2 in room temperature. You may add 10-15g potassium ferrocyanide per liter of bath as well. Benzotriazole acts as a brightener.
Thanks for posting. Nice clear explanation. I was trying to work out if I could copper plate galvanised steel. If you read this and don't know - watch the video!
Thank you! This explains a lot...
Shame I didnt find your video sooner. I started experimenting with electroplating and I was disappointed with my copper plating results and I couldnt find out why.
Other videos just mention that you need to plate steel with some other metal before plating copper, but dont tell you why... Or dont mention this stuff at all and poorly plate copper directly on steel!
I just tried plating a iron hammer head in copper. Total failure. Now I know why. Thank you.
I'm no expert but to nickle plate aluminium you must zincate it first also you can copper strike then zinc then you can nickle plate I would think the zinc bond on steel would be the same allowing copper to bond to zinc good video
Yeah, that's the only problem.
I've made the sodium zincate solution.
Plates well with a small layer of zinc, but I haven't found a way yet to make a flash copper solution to strike the metal.
@@neutronpcxt372 hi there any updates on your experiments to make flash copper solutions ? Direct to steel
I don't want to be like Karl.
To Chome plate steel part surface is cleaned and degreases, copper plated, then Nickel plated, then Acid Chrome plated. Copper plate in part to make the Nickel stick to Steel but Nickel plates with big "pores" or holes which allow corrosion.
This last image you showed is what I'm getting when trying to plate my parts with nickel it's like it won't adhere to the steel drive shaft which makes me think maybe it's not steel?
i gopper plated steel by using hydrochloric acid that had a copper bar dissolved in it, so it was copper in solution i then dropped in a super clean piece of copper hung form a bar over the bucket and the copper in solution adhered to the stell, i left it there for a day then took it out, washed it off and i cant rub it off, polished nicely bit im not sure how thick it is, I also did it by accident with my bricklaying tools and found out in the morning i had copperplated my dirty old trowel and float although by the end of the day the copper had come off the bottom, this led me to experiment further with a clean piece of steel and a bar of copper dissolved in the acid, that worked really well, its not electro plating but it certainly plated the steels and it hasnt rusted through yet.
mhhhh....you said: " i then dropped in a super clean piece of copper..." its a piece of steel - right?
@Garage Science, copper can definitely plate steel.
You just need to force an electrical potential right as you enter the steel part.
That will prevent copper metal from forming onto the steel, and will just plate the steel/iron nail with copper normally.
This doesn't work with stainless steel though, since you need to dissolve the stainless steel oxide layer first.
Meaning you want to zinc plate, then nickel plate, and then copper plate.
Interesting... I've tried doing it that way but it still rubbed off. Have you had success doing it this way? If so, how exactly did you do it?
@@GarageScience It can be done, but it needs to be done very quickly.
The first thing you need to do is clean the crap out of it. You need to wear gloves in this process too to prevent any oils going on the part.
Meaning you need to clean in with soap, then sand in using very high grade(500+) sand paper.
Then a chemical bath of lye(sodium hydroxide) can get rid of all the oils and the like.
If you want, you can use a dilute sulfuric acid bath to etch the steel a bit.
After that, connect the steel part to the negative lead of your supply, and the positive lead with copper wire.
Connect your power supply. Put in the copper wire first, then put in the steel part as quickly as possible. It will then plate using electricity, meaning no immersion deposit was formed.
This won't work with stainless steel, as it forms an oxide layer much much quicker than regular steel, like what aluminium does.
Also, if possible, put a bit of HCL in the copper sulfate solution. That will etch the steel, and allow the copper to fill in the gaps.
@@neutronpcxt372 good info, I'll have to give that a try at some point. Thanks!
@@GarageScience here's how I've succeeded in copper plating steel:
- clean the steel piece with alcohol or paint thinner to remove grease
- get a clean jar/container, put some 2/3 distilled water, saturate with salt to increase conductivity
- put some stripped copper wires and your steel piece in the container
- hook a power supply to copper and steel, give it around 3.5V and 3-500mA
- give it around an hour
You will see the steel piece get darker, it's ok, when you pull it out, use a steel wool to clean off, copper layer will NOT rub off, I've done this successfully to key chains and a small screwdriver for my digital calliper, works and looks great.
the water will contain copper sulfate, dispose accordingly
While copper plating steel is possible, it won't last. Plate with nickel first, then copper will stick. That is what I have found.
Yes. Flash plate with acid Nickel chloride first and then copper plate.
Hi sir
I have try copper plating but my copper plating is remove easly it remove by scratch with nail, then i was try with other iron plate, it makes black in colour, please guide us what is problem?
Just go for the electroless plating where you just need to activate steel first by dipping in H2SO4 before dipping it in a Copper Sulphate composite bath!!!
I can understand with just plain water but I have seen a video using acid water cs mix seamed to work . any thoughts on that
It's interesting, I used the same 0.5M HCl solution in an ultrasonic cleaner to clean an unknown copper alloy(brass/bronze?) followed by iron.
To my surprise, when I took the iron out, there were patches of copper securely bonded to the metal. I'll have to try it the future my deliberately adding a more concentrated solution, but I think the cavitation bubbles collapsing might give enough energy to weld the precipitated copper right on!
Is it possible to break up brass into copper and zinc. My hopes are that the copper forms on the electrode and zinc just falls down.
Oh but you can copper plat plain mild steel with copper, but not with straight up Copper Sulfate solution, I have done this with good adhesion and brightness . Try this formula copper Sulfate 10 grams per litter, 50 grams of Cream of Tartar and 50 grams of table Salt. no need for electricity.
Thats a very small amount of copper, with a small concentration like that you definitely needed something like table salt to add conductivity. I'd be interested in how thick of a coat you were able to get with that. The sodium in table salt would generally be considered a contaminate creating brittle deposits. If you send me some pics on the FB page I'll give you a bit of a shout out. Thanks!
@@GarageScience Sorry, I believe I mentioned that it's electroless. What I was trying to point out that using just copper sulfate you will not get a adherent coat on plain steel that is why you can rub it off with your finger , but using cream of tartar as complexing agent and Sodium Chloride as conductivity and chloride ions will give you a bright and adherent coating on plain steel, but it's a thin coating of course. there are old books of how to silver plate copper/brass using Silver chloride, cream of Tartar and sodium chloride. I substituted Silver with Copper.
@@GarageScience after this first layer u can use any other acid-based copper solution 4 thickening...
@@nicamarvin I have tested Marvins recipe (with electricity) - not really satisfying - but Its possible to generate a nice shiny layer on polished steel but adhesion is weak.
@@dieterkrause1674 yes it's quite dificult without Cyanid electrolytes.
Question: Zippo lighters are brass bodies, most of the time with a coating of stainless steel. What would be the most effective way to coat the stainless steel surface with copper? Would you need to first coat the steel with nickel and then copper? My goal is to have a fairly thick copper plated Zippo that I can then oxidize with a brass-blackening solution with 0000 steel wool. I’ve done this many times with the raw brass lighters, but I want to try it on a copper surface (without having to experiment on my expensive solid copper Zippo).
I've never heard of something being plated with stainless steel. If it has a polished white surface finish, then it's probably coated in nickel. If that's the case, you can copper plate directly to it. If it is, in fact, stainless, you'll very likely need to plate nickel to it first. I made a video on making a simple nickel plating bath.
@@GarageScience I concur with your assessment. I have worked in a plating shop for 17 years and what you suggest is correct.
So, for me to give an aluminum bath to an iron screw, for example, do I need to zinc it to create "adhesion" to the aluminum? Does it work that way?
The reaction video contained was copper onto zinc and copper onto Al. I've never done an Al bath. I honestly couldn't say for certain. Seems plausible that Al would deposit but the difference in electronegativity is more of a thumb rule than anything. Someone with a larger background in Chemistry might be able to chime in with a more detailed answer.
@@GarageScience I understand, I'm very grateful for your answer, where I live it's difficult to get this type of information but I won't give up. Thank you also for the video, it helped me a lot.
Thank you, this was enlightening
Nicely done.
I made some copper acetate with a pinch of NaCl, no idea about the concentrations, but I plated a steel cutter blade and it holds well (voltage was below 1V). The copper color turned black over two months. Not sure what it actually is.
thank you sir
So can you plate nickel onto zinc, and then copper?
Great explanation
Will copper plate to tin?
….. Um plating doesn’t occur to steel because you have to apply current to it in order for it to happen….did we miss some chemistry classes or?
have a look on zinc coated iron/steel parts...
Well, looking at the title, I have questions, because I am literally copper plating carbon steel right now (w sodium bicarbonate electrolyte that I dissolved copper into first... No sulfates) and it's been working great... Guess I should watch the video though heh.
Looks like you achieved a basic pH with which to plate similar to a cyanide plating solution. However the plating thickness is limited with this approach and since it's not a cyanide bath it's probably a brittle deposit with possible adherence issues. Check out this article for more info.
www.pfonline.com/articles/choosing-and-troubleshooting-copper-electroplating-processes
@@GarageScience Thanks. That makes sense. I went heavy on the bicarbonate. Also the steel was near mirror polished and cleaned with soap + water, then acetone, immediately before plating. Electrolyte was primed with all copper electrodes first. Also I ran it hard, around 10A at 30V maybe 4cm electrode distance, anode was a coil around part, holding electrolyte temp at 160-170F. I did notice a thickness limit. At some point it was just picking up loose copper or maybe red copper oxides, which were easily wiped away. The adhesion of the thin base layer seems decent, it survived an aggressive buffing wheel at least, and so far hasn't flaked away. My calipers aren't accurate enough to measure the plating thickness.
@@GarageScience I wish nickel wasn't so expensive... :(
@@ElTurbinado hey can you explain how you made your electrolyte? Baking soda and distilled water? How did you get aqueous copper? Or was just a copper anode required? Thanks!
@@electrowizard2000 baking soda and distilled water; heavy on the baking soda (it's not precise; just dump a whole bunch in while stirring until it stops dissolving readily). you can kind of plate starting fresh with just a copper anode but it goes more smoothly if you keep an excess of copper in solution. so what you can do there is first run it for a bit with a copper anode AND a copper cathode (the copper from the anode won't plate the cathode). run it until the electrolyte turns a nice blue (that's the copper). then swap your cathode out for your part, but keep the copper anode (so the solution stays copper-y).
at the end if you want to cleanup you can use two scrap iron electrodes until the solution isn't very blue any more. you'll still have dissolved copper but not nearly as much (some places don't want heavy copper solutions down the drain). or just save the copper solution for next time.
Is it possible to copper plate galvanised steel by using some other intermediaries? How would I go about doing that? (strip the zinc, then nickel plate and then copper plate?)
If it's plated in zinc you could probably nickel plate to the zinc. There tends to be an oxidized layer on the zinc that also helps protect the steel. Don't know if that would come off in the acidic plating solution but probably wouldn't hurt to clean the steel first in an acidic bath first before plating.
Can you please tell me how to plate a copper soldering tip with iron ??? When soldering the heat pitts the copper surface making soldering difficult. When the soldering tip is iron, this just does not happen. Making for a easy soldering job. Maybe make a soldering tip from nickel ??? Can you drop a line with your thoughts ??? Thanks a bunch too.
I have a video on nickel plating that you can check out. I haven't tried to perform iron plating so unfortunately I cant say much about that.
@@GarageScience Hello GS, I will check out your nickel plating fella. Please let me know when you are going to iron plate. Thanks VF.
Great channel could you go into the chemical makeup and proceedure for electroless nickle boron plating thanks for a great channel
Perhaps eventually. I'm working on designing a simpler acid copper plating bath (as compared to this one ua-cam.com/video/7QXi4PM39ho/v-deo.html ) and then I'm going to do acid nickel... then I may branch out to electroless plating solutions. I have links to other plating resources in my other videos that you may find helpful too.
Could someone explain why I experienced very good results and finish when I electroplated iron with copper in a sodium bicarbonate solution/electrolyte instead (using tap water)?
I was expecting poor results because many people online claim its hard to plate iron directly, however most who claim that are using a solution of copper sulfate. Would copper sulfate give different result in comparison with copper and sodium bicarbonate in this regard?
Dear Sir, what you said was very interesting. I have tried electrolyte baths of Copper acetate and Copper chloride. Both were acidic. Neither worked but quickly deposited a thick slimy layer of Copper that wipes off with the gloved fingers.
That can occur if the parts you're plating aren't clean or if the plating current is too high. Try lowering the voltage.
@@GarageScience Yes I suspect my parameters are too high. I have a fixed output transformer as my power supply. Time to invest in a variable power supply. Thanks.
I feel like Im missing something here. you say copper cant plate on iron because iron is higher in the the series. So use tin or nickel instead, but arent they lower than iron too, and isnt that the whole problem?
yes, acid copper plating solution wont work but you can just use an alkaline copper plating solution to plate directly onto iron and steel.
Many of those alkaline solutions contain proprietary blends to get good results and don't have as widespread open source knowledge. That's why I stayed away from talking about those. Cyanide copper plating also works directly with steel but is very dangerous.
@@GarageScience cyanide is obviously out of the question for amateur use but pyrophosphate or rochelle salt based ones are doable. but well, a nickel strike also works and is easy to do.
@@GarageScience Hi guys, I'm actually right now trying to make my Pottassium pyrophosphate and Copper pyrophosphate chemicals for plating from MAP (monoammonium phosphate) and KOH and CuSO4.
I need less corosive bath, since this pyrophosphate is 8-9 pH this is OK.
Since i'm early beginner in electroplating (I didn't even start properly) a have beginner question - Is i possible to coat tin metal with copper (pyrophospate bath ) and did anyone try this.
Do not be confused it is not tin electroplating, it's coating copper on tin surface.
Thanks, and great Chanel.
Yes you should be able to copper plate tin with that type of bath.
@@GarageScience Thanks for your replay I will try this very soon.
Keep a good work :-)
Doing resoration of vintage pieces like radio and bicycle parts. So I should nickel plate first then copper plate with brighners to fill in rust pits and scratches and polish before a final nickel plate. If you do automotive body work its kind of like putting on primer to make the body filler stick. Things are making sense.
hey dawg, can you plate wood? ya ya ya its poreos, its difficult blah blah blah.
nickel clay rubbed into the wood, or soaked in a solution that would deposit the metal.
maybe paint to fill holes and also apply a base layer? aka a metal paste
As long as there's a conductive surface, yes you should be able to.
Here's a Karl feedback... Copper plating directly on steel: ua-cam.com/video/oHSaUu2U06Q/v-deo.html
Isnt Iron or steel copper plated with cyanide?
That is another approach. Cyanide is extremely hazardous though and not for home users.
@@GarageScience thank You for Your answer: No risk, no garage fun;-)
@@GarageScience Cyanide is way overrated. It takes quite a visible amount of cyanide to kill you. Heck, you most likely have chemicals in your home much more toxic than cyanide, gram for gram.
@@GarageScience Don't be afraid of cyanide. It takes at least 400-800 mg to kill an adult by "intentionally" swallowing it. Several plants in my garden are way more toxic compared to cyanide.
I just have an answered questions
that explains everything i'm doing wrong.
thanks
as well as loosing my work piece and spilling $15 of nickle plate solution everywhere... its not a good night for this....