X-rays Photoelectron Spectroscopy - Surface vs. Ultra thin film vs. Thin film
Вставка
- Опубліковано 1 жов 2024
- XPS is a surface analysis!
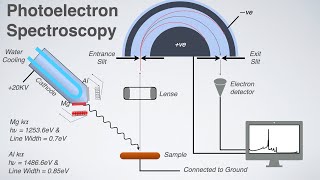
X-ray Photoelectron Spectroscopy (XPS) is a key and very important surface analysis technique. In this technique, an x-rays is simply bombarded on the sample, and the photoelectrons emitted from the core of the atom. The Kinetic Energy (KE) of the photoelectrons then detected by the XPS detector. Using the following equation, the Binding Energy (BE) can be calculated
BE = hv - KE - ϕspec
Where 'hv' is the x-rays energy (constant value), ϕspec is the work function of the spectrometer (a constant value). Therefore, the BE of the element can be calculated, which can reveal many important characteristics about the element. For instance, what element exists, what the atom or element bonded with, the chemical environment, oxidation states, whether the electrons removed or added to the atom.....
Question: Why is Studying Surface important?
Response: All materials contain surfaces, which interact with other materials. For instance, the key properties such as surface wettability, corrosion, adhesion, charge transfer, and CATALYSIS are all determined by surfaces, so surface analysis is important and XPS can be utilized to characterized the surfaces.
Question: What is Surface?
Response: A surface is an extremely thin layer, i.e., surface = 3 atomic layers (~1 nm). Size wise, the surface, ultra-thin film, and thin film are related by the following relation
Surface smaller than Ultra-thin film smaller than Thin film
Question: Why is XPS Surface Sensitive Technique?
Response: Approximately 95% of the photoelectrons are emitted from the ~10 nm depth surface. This is why XPS is termed as surface sensitive analysis. However, x-rays can penetrate to a few micro meter depths in the sample, but due to inelastic scattering and the smaller free mean paths of electrons, the photoelectron lost all of KE and can not reach the XPS detector
In XPS, x-rays completely transfer its energy to the core electron and there are Photogenerated electron, Auger electron, & XRF phenomena (this one is weaker and can not be considered). Therefore, the XPS spectrum contains only the photoelectron peaks and Auger electron peaks.
In the XPS spectrum, the number of electrons detected are plotted on the y-axis while the BE plotted on the x-axis.
Secret Behind "hv = BE+KE+Ø" Equation for X-rays Photoelectron Spectroscopy
• Fundamental Equation "...
XPS vs XRF vs Auger Effect- X-rays Photoelectron Spectroscopy
• XPS vs XRF vs Auger Ef...
What is Binding Energy (BE) in X-rays Photoelectron Spectroscopy (XPS)?
• What is Binding Energy...
Importance of Survey Spectra in XPS - X rays Photoelectron Spectroscopy
• Survey Spectra in X-r...
Why p-orbital, d-orbital, f-orbital have TWO Peaks- Doublet in XPS Spectra
• Why p-orbital, d-orbit...
Why XPS is a Surface Sensitive Technique?
• Why X-rays Photoelectr...
Why Only Core Electrons Peaks in XPS - X-rays Photoelectron Spectroscopy
• Why XPS Only Analyze C...








Dear Sir, I'm watching Thank you very much for uploading xps video
Great to hear. Please do comment if you have any confusion.
Dear Prof. thanks a lot