Liquid Metal Embrittlement - Gallium vs. Aluminium
Вставка
- Опубліковано 28 вер 2024
- Bringing gallium and aluminium together has some interesting and occasionally rather explosive results, as Dan found out in this little tale from the prep room.
Subscribe for regular science videos: bit.ly/RiSubscRibe
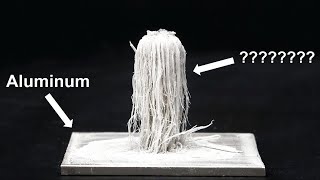
Liquid metal embrittlement is a phenomenon where ductile metals, like aluminium, become brittle, when exposed to certain liquid metals, like gallium. The mechanism by which this happens is not a chemical but a physical one, where the liquid nature of gallium allows it to get into spaces within the aluminium structure.
This embrittlement of metals is real a hazard in manufacturing industries as well as other fields where ductile metals may come into contact with liquid metals.
---
A very special thank you to our Patreon supporters who help make these videos happen, especially:
Ashok Bommisetti, Avrahaim Chein, Greg Nagel, Lester Su, Rebecca Pan and Will Knott.
---
The Ri is on Patreon: / theroyalinstitution
and Twitter: / ri_science
and Facebook: / royalinstitution
and Tumblr: / ri-science
Our editorial policy: www.rigb.org/ho...
Subscribe for the latest science videos: bit.ly/RiNewsle...







![[593] Gallium vs. Titalium - Abus Padlock Meets a Gruesome End](/img/n.gif)
It looks like the exploding can was the only one sanded completely. Not only did this reduce the thickness of the can, but the other cans look like they still had their full labels, which aside from the paint may perhaps even include some sort of clear coat. Either of these will inhibit your attack surface.
You are on to something. pressurized, but shake it and you got a fountain then opens. And you then shake it while sandpaper. The fact the other cans were not sandpaper mean the pressure were far less in them. Combined with a damage surface, maybe caused it.
also notice the scratch he did with his knife on that one much bigger area using the blade long ways than the scratches before
the sanding would also make the can thinner in spots. because im sure it wasnt sanded evenly in all places
I think the can is thinner after sanding as well, so once the can is weaken by the gallium the pressure over come the structure strength and explode
Thinner surface, probably the can itself was hotter from being handled, and was in a warm environment, was on the high side of internal CO2 gas fill mass, along with being shook up. All increased the internal pressure, and you had both the right thinly drawn can ( probably a brand new drawing ring set, giving the thinnest metal from the die), the grains being all lined up with the drawing stress to give long fracture lines, and enough internal pressure so that as it fractured there was still enough pressure to propagate the crack along the can giving the burst.
In 'slowmo' the tear is shown in two stages, first halfway, then the pressure of the coke does the rest of the tearing. You also smeared the gallium out a little bit. Things different on other run: 1) Precondition/storage: shaking? hot lamp? storage? 2) Warmth of your hand at the bottom of the can, non-conductive (no grounding) 3)
salt on finger? 4) straight tearing indicates weak welding line? 5) Sanding (removing label) of the can?
the can was shaken, not stirred
I like my soda exploded with gallium not shaken or stirred
my guess is that the gallium was mid-can. the can was tilted allowing the co2 in it to rise to the top. it weakened below causing the co2 to expand. liquid rushed out first with more force. when you did the experiment again the cans were flat. the point of weakening was exposed to the co2 directly which was far less explosive. but that's my guess not fact ;D
That phenomenon is really amazing.
Now that was interesting
The pressure (co2 gas build up) in that particular can was more the others (probably from being dropped), any weak areas will allow that pressure to be rapidly released over small area and hence explosion
The can in your hand had been disturbed more immediately before application of Gallium, and it was also warmer than the other can. Higher internal pressure could be the explanation for the difference.
I think you created presure in soda can by shaking before applying liquid gallium. It pops strongly because little hole due to gallium causes it to burst.
The exploding can was sanded, whereas the other times you tried, you used a can that had the paint still on it. Try it again with a sanded can!
I imagine when the can was sanded, the drink inside got shaken around a bit and pressure built up making it explode when the gallium weakened it.
If you would heat up the aluminium would the gallium evaporate so the aluminium would become hard again?
That one can probably just has a problem with premature exploderation?
Did you shake the first can a bit maybe just before? I mean, you were literally handling the first can while the rest were placed.
Temperature of the cans would be my next guess.
Compare the aluminum grains to those in copper..which has weaker metallic bonds but stronger grain boundaries.
Was the first can shaken up at all?
maybe shaking the full can before applying gallium?
Well, it's "completely" safe. Don't go drinking the stuff. It's still a trace heavy metal.
and thats why you arent allowed to take a mercury thermometer on an aircraft , or gallium
Safety squints on? Oops @ 1:45 ...
Don’t spill Gallium on you airplane? While in-flight?
Yeah, it's hard to clean up. But it needs to have clear access to the pure Al that's under the oxide layer for this effect to happen.
Ga and Hg are strictly forbidden from being transported on aircraft for this very reason.
Do they have gallium and mercury sniffer dogs?
Oh no there is gallium in the rain, flap your wings birdies.
but hey, still not as dangerous as nail clippers
1:29 is a reminder to always wear safety glasses!
It looked like the one that exploded was sanded? Faster and more intimate contact with the aluminum, but also the sanding scratches might have served to concentrate stress and provide an easier way for the tear to propagate?
explanation is too simple, why don't other atoms penetrate another atoms like gallium does with aluminium?
The one that exploded must have been shaken alot during the sanding and was under pressure, so it exploded when the metal got brittle. The other ones weren't sanded and not shaken (presumably) and therefore didn't show this drastic reaction?
The sanding could produce heat, increasing temperature, too.
And the shaking could be from holding it, given that it was in his hand while the others weren't.
It wasn't Gallium but a droplet of mimetic polyalloy. Sent from the future to destroy humanity.
How do you get the gallium back
Could you destroy an entire aircraft with just a few grams of gallium?
In the sense that it could suffer a structural failure and crash; yes.
In the sense that it could suffer a structural failure and crash; yes.
In the sense that it could suffer a structural failure and crash; yes.
In the sense that it could suffer a structural failure and crash; yes.
I'd take a guess that the sanding that was done to the can, that exploded, may have caused more CO2 to be undissolved in the cola, since the others that were shown were not sanded.
If that was the cause I would also want to test if sanding the can axially instead of vertically, or vise versa, would cause the split to be different.
shake the can? the crack went from top to bottom you must have scratched the Al to form a microfracture that expanded when the gallium cracked the center part due to internal pressure
The first can you were holding in your hand, warming it. Not only will this have increased the migration speed of the gallium through the aluminium, but importantly would have raised the pressure by CO2 tending to evolve from solution. Try warming the can to 30C and trying again...
Nice, right to the face without eye pro. Probably in your eyes and mouth. You should season your food with cilantro for a few weeks. Poor mans chelation therapy, good for you every once in a while anyway.
My guess, for what made the o e can explode, is the handling. Leaving it and letting the gallium work its own way, led to a small fissure. But handling the can, shaking it and raising the pressure made it fail catastrophicly just before the gallium reached the inside, so a bigger gap could tear open through the thin layer of still uncompromised aluminium. Am I making sense?
Probably, the exploding can had been shaken before you did the experiment:)
Fast fracure (as in the explosion) occurs as a result of an applied stress and a defect of a critical size. In this case the stress is provided by internal pressure, the defect being the galium-affected zone of the aluminum. In the painted bottles this zone is small, since the paint prevents contact with the liquid. In the sanded bottle this zone is bigger than the critical size for fast fracture.
Cans are pressed from flat sheets of aluminium, there isn't much science behind the process as they don't need to hold much pressure (in comparison to the manufacture of bullet casings or high pressure gas bottles for example), some sheets my have a much higher density of grains than others just from slightly different cooling times and rolling process (engine turbine blades can be made from a single crystal, no grains at all so much stronger in general). Not only will there be a fairly broad tolerance on the thickness of the can walls, the pressing process affects the grain boundaries, stressing the bonding between them before you even start messing with them with the gallium. The method of removal of the cans print and the oxide is full of variables, from how much the can is shaken to how much of the ink/oxide is removed The difference in strength from can to can, added to the increased surface area from the removal of the ink and any any oxide. Basically, there are so many variables it's impossible to know for sure if the can is going to immediately explode or take it's time and just slightly rupture.
Maybe the can that exploded was being warmed by your hand and under slightly higher pressure?
Or maybe you were holding it 50cm from your face and not wearing eye protection and science decided to scare you into remembering that you were following bad laboratory practices.
I think the can might have reached room temperature that means internal pressure is increasing because CO2 is coming out from the liquid so just little bit of destruction of Aluminium’s lattice the gas trying to come out has broken it causing an explosion. More the internal pressure the speed of the explosion is very fast. Because coke, Pepsi etc are recommended to store in a cool dry place.
i think so tye main reason why can exploded was that the can may be pressurized before when u take it means it may got a jerk and get pressurized and when u put gallium jt gives it a path to blow out through the can
You left the paint on your cans in your later experiments and that may cause the Gallium to spread less easily, preventing the cans from exploding. Maybe you can get the paint off the can and give it another try.
I’d like to see this happening with an old engine whilst it’s running, it’d take longer but the failure would be interesting to see.
Was the exploding can shaken prior to adding the gallium? What temperature was the can that exploded vs the cans that didn't? It has to be pressure, the can that exploded ripped open where the other cans simply punctured open.
The labeling material would have acted as a lamination layer providing more tensile strength than the can which had the label layer removed. Like laminated bullet proof glass.
but if gallium can penetrate, why water does not..?
the aluminium cans are extruded from an aluminiumn disc. maybe the extrusion process left a weakness in the material and when the gallium was applied the weakness was not longer strong enough. you can see that the material was ripped apart from the middle to both ends in a straight line. i think the cans are extruded in this direction.
guess on why the can exploded: if the sanding scratched a line down the height of the can, that line would serve as a defect for the can to split rather than just puncture, much like scratching a line on glass gives it a direction to crack along.
Think the explosion was due to the aspect ratio of the scratch. It seems to me the one that exploded had a more linear scratch. This creates non uniform stress field with stress concentrations at the ends. These stress concentration move ahead of the crack tip allowing it to propagate at close to speed of sound for the material, giving the crack time to grow before the pressure in the can is released. A low aspect ratio scratch will produce a circular hole with no substantial stress concentration allowing the pressure to dissipate without further cracking.
Is it because of dynamic embrittlement that lead to a tensile force sepearating the grain boundaries and same time ga liquid penetrating between the grain boundaries ?
My guess is that because the can that exploded had been sanded and the others hadn’t, the sanding and associated shaking action done by sanding had pressurised the liquid inside, therefore the sanded can had higher pressure and the liquid wanted to escape faster, hence the explosion.
"Chemical metal embrittlement!"
I see what you did there..
When your parents lock you inside of a basement and the door is aluminum then you can easily get out if you have some gallium on you
Can you recover the Gallium from the Aluminum?
so is it right to say that liquid embrittlement is a intergranular corrosion?
Cans have a seam...maybe the exploding can had gallium right on that seam, making a tear that would propagate along it.
Aluminium cans don't have a seam along the body, each can is extruded from an aluminium disc that then has a top added, the only seam is round the top.
ua-cam.com/video/hUhisi2FBuw/v-deo.html
Oh, I didn't know that. :) I thought they were made in a similar way as tin cans. (Which doesn't make sense now when I think about it.)
The paint interacted with gallium somehow? Just guessing...
SCIENCE IS SO COOOOOOOOL
We agree.
The first can had the plastic label sanded off.
Was the can shaken before experiment?
Temperature of the can. How bout shaking the can? Lol
But what about the music, its so good whats its name?
Why does mercury not do this? Or does it?
Can I use this to destroy an aluminum seatpost stuck (well stuck) in a steel bike frame?
If I scratched the inside within a screwdriver or some sandpaper would that help?
Is paint safe?
Don't do it, the Ga has this effect on steel too.
Maybe the ink or paint on the cans?
Sanding the whole thing by hand prior to filming probarbly not only weakened the surface, maybe enabling long fissures to form more easily. You may also have throughly shaken up the contents, which temporarily increases the pressure, but also spreads around small bubbles that make things much more explosive by suddenly turning into larger bubbles as the can fissures, releasing more CO2 from the liquid. So try sanding and shaking. Also making scratches with a blade seems rather crude. Can you add some abrasive, like corundum sand, into the gallium and then rub it on using a Q-tip or something?
I'm assuming you guys shook the can to get an explosion? Maybe the pressure when shaken is too strong and therefore jets out like it did? Rather don't shake the can?
We didn't deliberately shake the can. It may have shaken around a bit during the sanding though? Something to consider.
What was different is the sphere of oxygen influence that surrounded the can. The first experiment was not contained in a box but the room itself and all the rest of the shewn experiments were contained in a smaller area.(the transparent container/box)
However I know not for sure it is just my observation.
You sanded the can down.
Was the can shook before and was it the same brand? Also it looked like it split open down the seam of the can maybe placement is key.
We thought so too, but then someone sent us this video - ua-cam.com/video/hUhisi2FBuw/v-deo.html - and it turns out that aluminium cans don't have seams on the side. All the cans were of the same brand and none of them were specifically shook before the gallium was placed there. However, some shaking may have occurred through the action of sanding so maybe there's something there?
My guess is that the scraping of the paint off the can was too aggressive and made a weak spot that ended up exploding, while other cans weren't scraped as much and remained strong.
When a deep diving submarine goes beyond its pressure depth the water passes straight through the steel as a mist.
Hi James, this is an interesting comment. What depth was it at?
This is a crazy question but did the can weight change over night?
Well you had removed the paint all around the can that somehow fastened the degradation of the aluminium bonds they got compromised faster, almost as aluminum had liquid properties?. Droping paint in water was what i come to think of.
For a crack to grow, the energy taken to drive the crack forward has to be paid for by the release of stress in the material, this gives rise to a critical crack length, being the minimum length of crack needed for this stress release to 'pay off'. It looks to me like in the slower burning cans, you made a small incision, which may have formed a crack below the critical crack length - meaning the can didn't explode, and allowing the pressure to be released - meaning the can definitely didn't explode! Comparing a small incision to the wide weakened area that would form from the gallium, I can imagine that first crack being larger, and not allowing the pressure to be released.
The phenomena is called 'Leak before break': www.fose1.plymouth.ac.uk/fatiguefracture/tutorials/FractureMechanics/StressIntensity/KTheory4.htm
Though have to admit it's been a while since I studied this stuff!
The bursting can... It could be as simple as that particular can having been physically aggitated more. Either a happenstance of non-deliberate conditions or deliberately done to make the effect on film more exciting. If so, a plan dropped for subsequent tests, or more care was taken in handling.
A more complicated explanation, is that the amount of dissolved gas in the liquid is dependent on many factors, like the pressure in the can and the temperature of the liquid. So it could be that the conditions in that can were just right to make for a higher internal pressure in the can, which caused a more impressive failure.
Also, every can will be different on a microscopic scale,and even a macroscopic scale as no mass manufacturing process is 100% consistent, nor would your label/paint removing process be consistent between cans. Sanding can leave long scratches of possible significant depth. (I work in Aviation, and we are not to use metal scraping tools to remove sealants and paints on the aluminum skin of an aircraft, due to vibrations causing stress fractures to form if you sratch the metal with your tool, and it can lead to structural failure that can be catastrophic on pressurized aircraft) This difference in structural strength could result in a larger area of weakness in the can, and could allow for the pressure release to be larger as it tears a larger hole in the can, due to the already present structural weakness being amplified by the gallium embrittlement.
Awesome their honesty by being genuine admitting that "the cool explosion" only happened once for some reason they have no idea. Most other channels even would have caused that on purpose because "it sells more" at the cost of giving a false impression of the phenomenon.
Safety glasses please!
You shook the sanded can by sanding it roughly. The pressure was released quick enough to rupture the can. Maybe their was a small dent that split readily under pressure?
I guess you only sanded the exploded version and therefore the pressure due to the intense shacking was for the time much higher in the can. Also, you sanded a large area an not all was covered by an aluminium oxide layer immediately. Maybe a layer of your sanding material was still there, and the galium sneaked in.
Hi! Cool video. We want to do the same experiment for school and wanted to use 20 grams of 99.99% Pure Gallium Metal, will this work as well? Grtz
You probably won't need the full 20 grams and it should work, yes.
@@TheRoyalInstitution thanks for the response!
Where can I get gallium?
on Amazon
I found several sources via a Google search for 'gallium for sale': www.google.com/search?q=gallium+for+sale&sa=X&ved=0ahUKEwj6v9iSqszcAhWTxIMKHSQTAH0Q1QIIgAIoAQ&biw=1280&bih=677
Ebay: www.ebay.co.uk/itm/Gallium-Ga-99-99-pure-metal-element-31-nuggets-ingot-10gr-50gr-suppliers-kg/123197195115?hash=item1caf20036b%3Am%3Am8Bggp8T9zWH5anqAmsiqAQ&var=423604628892&_nkw=pure+gallium&rt=nc
Maybe something to do with tem of the can
The Galium exploded can was held in the hand. Possible temperature, pressure or interaction with oils from extended hand contact changed things... or maybe some one just dropped the can at some point. Cans sometimes splurge when you open them.
If the sanded can were the only to do this a couple of plausible reasons come to mind, perhaps a combination of both. The sanded surface obviously allows for better contact between the metals causing a faster reaction. A larger area is weakened more quickly, allowing for a more rapid release of kinetic energy. Secondly, the surface sanding will agitate the soda, causing more gas to come out of solution than an undisturbed soda. The gas out of solution will increase the pressure inside the can, providing for a more dramatic demonstration.
Thanks, man. That was ahhmaze ing!!!
Would gallinstan (a low melting alloy of Ga + In + Sn & a non-toxic mercury substitute) have a similar effect as Ga alone?
Might the cans that didn't explode have some sort of polymer lining that helped to handle the strain and keep the grains of aluminium together? It's hard to say without details on the bottler and their production methods, but I think linings are fairly common these days.
How long did you wait after sanding the can before trying? Seems to me you inadvertently shook it up and increased the pressure.
It was the only one you were holding at the time. As you showed, once LME starts you need barely any force to break the metal. The small amount of force from your hand putting pressure on the can to hold it in place was enough to make it "explode"
When you had the can in your hand, it was tilted so the gas was in the top and the weak part had liquid behind it, making it explode. When laying it flat, only gas will escape.
When you removed the paint from the exploding can you work hardened the aluminium making it slightly more brittle. You also used a different scratching technique that removed a larger area of oxide.
The can exploded the first time because the gallium got to penentrate more surface area before the soda was able to put preassure on it
Cool
why doesn't this happen with with other liquids like water?
great video btw
There appeared to be a pressure difference in the first can, and there my have been a temperature difference as well. Was the can shaken, or was the can heated before the experiment was performed?
The temperature of the can and if it had been "shaken" or extra energy between the liquid soda and the gas carbon dioxide.
It's also used as a high end thermal paste (thermal interface material) for high end computers.
4:11 The ink on the unsanded cans was protecting the aluminum against penetration of the gallium.
It's a great video, thank you ... can you do it with iron metal?
I reckon you might have mixed some powdered menthosin with the gallium 😂
Mercury has a similar effect.
This one needs a super slow motion recording. Because SCIENCE!
Who knows, if our Patreon page takes off, maybe we can invest in one of those Phantom cameras that the Slow Mo Guys use...