How to Find Theoretical Yield (2023)
Вставка
- Опубліковано 10 гру 2024
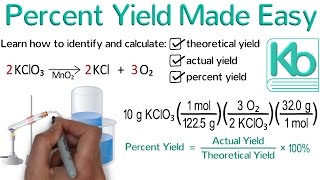
- Convert all amounts to Moles
Divide all moles by the COEFFICIENT of balanced chemical reaction
Whichever of those results is lowest corresponds to your LIMITING reactant
Use that reactant's number of moles and a MOLE RATIO to figure out amount of product made
Convert to grams if necessary








Explained this better in 5 minutes than my professor did in an hour
So true
Really
An hour,the whole year!😢
I don't know what to say, but thank you SOOO much!! You have NO idea how stressed I was because of chemistry. I couldn't study the entire year and i had only a month left till my exams and i binge watched all your videos and I absolutely aced my chem exam! THANK YOU!!!! You're so underrated, no one else explains topics and solves chem problems like you do. Your way of teaching helped keep focused. You also don't out all your knowledge behind a paywall like most people do.God bless you ♥️
Im an A-level AQA triple science student, so you can guess just how grievous my situation was ahaha.
How did it go?
@@honeydew1411 Passed 🥳🥳 top of my class.
@@abcdefg-hv2ksgood for u my guy. Glad ur finally stress free 👍
I watched 10+ tuts, and din't get any shit out of them, this explained the concept in less than 6 minutes, W professor.
Watch Kevin maths and science he's better
It's the day before the last day of school. I've been stuck on the last problem on my portfolio assignment for hours. I couldn't figure out theoretical yield. I watch this video and in 5 minutes and 21 seconds I finally understand. Thank you very much for this video and teaching me a problem that closely resembles the one I have to do. Thanks again!
you explained this SO clearly, i finally understand THANK YOU !!
What a great UA-cam find. 💯 Thank you for your services
Understanding the yield calculation was so easy. I never even thought about it before.
I watched so many videos and could not understand how to do this until I found this one! Thank you so much!
Hey guys don't think deeply, people who work in pharmaceutical it's very easy to find.. Molecular Weight of your product/Molecular Weight of Starting Material*Batch input(Theoretical yeild), Batch input/Theoretical yeild*100= Percentage of yeild
for some reason theoretical yield is a concept i was NEVER able to wrap my head around no matter how many times it was explained to me. after literal and actual weeks, THIS is the video that's finally managed to drum it into my thick skull. Thanks man
Thanks dude! I was confused by this for years and you made it so simple.
Thanks sir for uploading such an amazing vid
You can't belive how much did your video help me... This subject has too many videos explaining too many different useless ways of solving something as simple as you just put it. Great job, you saved me!
EDIT: I have a quiestion though... what do I do if I am not given the masses of not the reactants but only of the product?
Watch Kevin maths and science he's better
Perfectly clear, thank you.
Magical video 📸
Thank you thank you thank you👍👍👍👍👍
You may have just saved my grade, and for that I thank you ✨
thank you allan from smiling friends i appreciate your help
You’re saving me 🙏🙏🙏
W video I understand it very well thank you
Thank you so much for this!
I would recommend Kevin maths and science he explains better
Thank you!!best of luck!
Great Explanation ❤
The explanation was great, I finally understand it. Thank You. BUUUT, where did you get the initial 3.0g for 2H2 and the 29.0g of O2. Can you please explain? Thank You,
It's part of the question
Great explanation this is the only video that’s helpful for me
you rock. thank you very much
❤good video
Best xplantion of all time for theoretical yield I also want actual yield how to find it
Really great explanation!! Thanks so much❤
very well explained!!
Thank you so much!!!!
Thank you:)
Thank you sooo much
Uve really helped me tnx a lot
Very helpful thank youuu
Thank you man😊
what if you not given the mases of the reactants only one?
Then your screwed
Then the reactant about which information is given is your limiting reagent
very useful! Thanks a lot
Thank you!!!:)))
Angas gagi HAHAHAHA
Thank you
genious!
I appreciate how you have presented and simplified this concept ❤....but I a little bit don't understand on the part where you are dividing number of moles by their respective coefficients😮
love it
Thanks for the forecast! Could you help me with something unrelated: I have a SafePal wallet with USDT, and I have the seed phrase. (alarm fetch churn bridge exercise tape speak race clerk couch crater letter). Could you explain how to move them to Binance?
Good video, bit wanted to ask that what if we don't know the mass of one reactant
Thank you so much for this amazing video! A bit off-topic, but I wanted to ask: My OKX wallet holds some USDT, and I have the seed phrase. (air carpet target dish off jeans toilet sweet piano spoil fruit essay). How should I go about transferring them to Binance?
Damn that was faster than the other formula and much easier
thank you!
You might have just saved my chemistry exam😭
Thank you so much🥹
Appreciate the detailed breakdown! 🧐 Just a small off-topic question: 😅 I have a set of words 🤷♂️. (behave today finger ski upon boy assault summer exhaust beauty stereo over). Can someone explain what this is? 😅
Among the reaction which reactant are we suppose to use for us to find theoretical yield
Isn’t the molar mass 2H2 4?
Or are we ignoring the mole?
How would I find the percent yield with this kind of equation, where neither PY or actual Yield is given. Would the AY be molar mass of a product - the amount of product lost?
But why dindnt you multiplythe 2 of H2O by the 1.5 of h2
Yes! Wonder why this wasn't the case?
@chemistNate what happens when you three products
Wouldn't o2 be limiting cause it's completely used up
I tried doing this with the net ionic eqn but it didn't give the same mass of product in the end. Strange
It's for a quiz retake im doing with the reaction between 0.040 moles of Silver I Nitrate and 50.0 ml of 0.60 M Calcium Chloride.
❤🎉
❤❤
Couldn't understand better
How did you find 3.0 grams from hydrogen
it was given in the question
where the hell did you get 18.0 grams from?
The molar mess of H2O
i love you
W mans
How'd you explain an hour's worth of lesson in 5 minutes
❤❤❤🇵🇬🇵🇬🇵🇬🇵🇬🇵🇬
Wa Ter
Unhelpful