What's the Difference Between Molarity and Molality?
Вставка
- Опубліковано 28 вер 2024
- To see all my Chemistry videos, check out
socratic.org/ch...
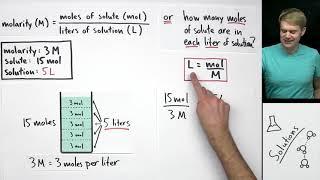
What's the difference between molarity and molality? They are both measures of concentration, but molarity has to do with volume (in Liters) and molality has to do with mass (in kilograms). Also, molarity talks about the volume of the total solution (solute and solution combined), and molality is about mass of the solute and solvent separately.







i think my teacher needs to watch this video
Lol...
I m your teacher !😑
U right
😂 even my teacher
Actually my teacher watches taylor’s videos
Hey everyone, I'm here to help. If you have any questions or just want to learn more, click on the link in the description above. It'll take you to a page where you can ask me questions.
Tyler,
I want to thank you in name of all my students for your great videos. Our school, like most high schools, had to go to distance learning during this corona virus pandemic. While preparing my lessons I was so happy I could depend on your videos for excellent instruction.
You always explain concepts so well and detailed that I feel my students will have an excellent foundation for their future chemistry classes .
Clearest definition of Molarity and Molality I've found! The visuals help so much
Yes, you're absolutely right. But usually that difference is so small that it doesn't matter, even in solvents of different densities. The one situation where it does really start to matter is in solutions that have a very high concentration of solute, so if you're dissolving a whole ton of something into a solvent, then that can really start to add up.
3:48 - 3:49 That's what I was thinking in chemistry class, and that is why I have to watch this video.
HAHA
Been subscribed to you for so long now, I've stopped since my A-levels but I'm preparing for a prelim test and I'm recapping. Thank you so much for the videos you've posted. You've got a knack for teaching!
You are my Chem savior! My college professor's explanation was not even close to yours. You should host lectures where you teach professors HOW to teach these topics.
your lectures are conceptual & easy to understand.
your videos helped me so much to understand chemistry
from 2020 and still love this video
Please make a video about Normality and Formality
That was so easy and understandable tysm 🍮
Only good teacher can teach like this
ur teaching is very easy to understand! LIKE!
Thank god, your a blessing man I was struggling on my ap chem work and because of you it’s easy
our a wonderful instructor, professors should watch your videos and learn from you so they can teach better. Thanks for your wonderful videos....But why you didn't come up with videos recently. We like to see you soon on UA-cam....Thanks a lot Sir...
So if I'm interpreting this right, there should also be another very slight (in this example at least) difference, but still important. In a 1M sol'n there is 1L of CaCl2 + Water, which would weight out to basically 1kg. However in the Molality it will actually be 1.111kg, which is 1kg of Water + 111.0g of CaCl2. Is this correct?? This would no doubt become a more significant difference also in different density solvents.
This man is so much better than my teacher, who is being paid a salary. This guy works off of add revenue
NOW I GET IT THANK GOODNESS
I'm sorry I forgot to say this, so the correct term would be the molar or molal concentration of the solute (in a solution), right? And one more thing, so we just assume the entire amount of solute is dissolved, right? Like there isn't any part of it that is not dissolved, since the definition of concentration is the amount of solute dissolved in a solution. I hope you can answer my questions, thank you.
God bless you sir.
Boss/Sir/ you own my heart and I can't seem to understand how to thank you. You exactly explained how I wanted to ❤️
so is Molality ALWAYS greater than molarity in aqueous solutions, or are there factors such as density that will change that ?
Wow this is so clear to me now, thank you!
You are THE BEST .
I am loving it learning
Create for organic chemistry also sir
Plzz upload Normality and Formality
I'll definitely want my teacher to see your videos. This is how science is taught. Not like how my teacher does. 😋
but can you make a video on solution and suspension
Thank you so Much sir you're awesome........ Love from India
that one hot chemistry teacher
bruh
That student who is only here for the bod. I mean seriously?
Do you think you could make a video on molality practice problems?
Keep going
You my friend. You. You have saved me from a date worse than death, and you will be rewarded in the afterlife. Peace be with you
You are so smart Man 💯
Sir u are great
Sir in ur country which class study this chapter if u dont min can u answer me
So molarity is basically volume and molality is weight
Also at 2:11 shouldn’t the moles be in grams or am I buggin???
wow....amazing...thk u..
Thank you for your video.
thanks bro! helped me alot..
thank you so much
great
cant you give more vidios on topics like #NORMALITY...
and the chapter #CHEMICALBONDING
thank you sir!
videos on quantum number ..
Thanks Babes
THE BEST!! !!
well that is truuue for water BUT it's not true for MANY OTHER solvants
i wish u can be my teacher
9 years ago 🤓
You
Save
Me
does no one else notice concentration is spelled "conceutration"??
Only m here who thinks that He looks more like "Justin beiber"😄😂😍😜😎
👌👌
oR ……MoRaLiTY.
All Hail Are Lord And Savior, Tyler DeWitt!
how many IIT-
JEE aspirants here
Hamara alakh pandey hi theek hai yaar
😂 11 years ago 😅
WTH hits the thumbs down button?
haha i thought my teacher made a typo on his worksheet when it said "molality"...
oops :)
thanks so much for your kind comment! i'm very busy and can't promise that i'll make videos on certain topics, but i always consider requests when i'm deciding what topics to address. so please let me know what might be helpful for you to see.
Normality?
Can you please do a video on normality
Mole Fraction
Please take your job as a tutor in our school in India....
Precisely Tamilnadu.....
but then what's morality !???
runetude i don't know why but this comment gives me life
:
The amount of unnecessary prejudices and superstitions one has acquired [on top of obvious basic decency ]
/ per unit of brain matter
...[ ? ] possibly.
This ratio is expressed in arrogance and measured in dB.
LOL!!
hahahaha!!
Beautiful!! xD xD
I FUCKING LOVE THIS GUY
Hey Tyler, I created an account just to tell you this. I have been reading about mole (your other video) and molarity and molality for more than 20 years (I am a physician). I never had it explained this easy. You are awesome!!
Aklilu T/Michael I am also a Doctor 👨🏽⚕️
@@cloutgangster I'm doctor octopus
@@cloutgangster stop capping
UA-cam: NEVER TAKE DOWN THIS VERY CLEAR & IMPORTANT VIDEO!
This guy....is saving my life...this and the gas laws and all that...helping me for passing the exams...Thanks a lot! :)
But which is greater...Molaltiy or Molarity?
Molality does not change with temperature but molarity does
Shouldn't it depend on the density of solution and solvent?
@@srirampatnaik9164 no it depends on volume
Your a wonderful instructor, professors should watch your videos and learn from you so they can teach better. Thanks for
breaking it down and making it simply =)
Tyler, you are the best Chemistry teacher! I'm doing well in my Chemistry class thanks to your videos.
can you please make a video about Normality?
I asked him the same in other video :D so 2 votes !
Yes yes an English video about normality pleeeeeese!
being normal is incredibly overrated tho.
I dunno how he can be so incredibly a great teacher
Exceptional explanation. It made things very clear.
Thanx Tyler for helping me in my review of my science/chemistry test
(#PRAYFORMEGUYS XD)
يا شيخخخ احوب خااالتك 😘😘😘💖
Damn you scientists for SUCKING at naming things.
My turn:
Moles Solute / Liters of Solution = MoLiSol. MolaLi. MolaLity. MolSolurity
Moles Solute / Kilograms of Solvent = MolGraSol. MolaKi. MoGrarity. MolSolvency.
My names have CLEAR OBVIOUS differences to avoid any confusion, and are related to what they mean.
Lmao 💀💀💀
What is normality then ?
Being normal.
one more thing, the reason some chemical equation preferred molal over molarity is that molality wont be effected by the temperature. the heat that is generated towards the solution will make it's molecules expanding therefore the volume increased, but the mass remains the same
Tyler, thank you so much for your channel. I am a mature student that is trying to upgrade his high school credits online, through community college. If it wasn't for you doing this channel, I would NEVER have been successful, I owe you a great deal of thanks.
Excellent!, Chef Kiss🤌. Thank you
goddamn ur a lifesaver
How could all these 23 people dislike it
Ajit Singh 38 ppl now😬
Hi, i just wanted to say that i seriously love your videos and i was worried that you had stopped making them because i hadn't seen any videos pop up in my homepage for ages! I am in a vital year at school this year and you made this video just in time! please reply to my message so i know you have actually read it because i have a few requests if it were possible for you to do.
Best until today 🤗
You saved my life
Sir you are the best teacher.👍
can you please make a video for solubility??
Please make a video on Normality, Equivalent Weight and n factor. Thank you for the interesting videos that you make.
Lol i just realized that whenever im making a juice drink using powdered juice, im using the molarity side rather than the molality😂
even ur eleven year old vid is better than my teacher explaining 3hrs for this simple topic in this era of digital board and facility (i wish ur were born in india )
hey u in 11th grade?
Tyler is a professor
Thank you 🥺❤
Your video helped me so much
you're really good instructor and professor 👍🏻
Been encountering this lesson in junior high, in senior high. I'm now in college and here i am again lol
same, got my uni entrance exam tomorrow 😹 learned this in 10th grade
So helpful
I really love the way you teach!!❤❤❤
Thank you
This man right here is the truth